
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Ronald B. Brown | -- | 1537 | 2024-03-12 13:39:05 | | | |
| 2 | Peter Tang | -1 word(s) | 1536 | 2024-03-13 02:01:08 | | |
Video Upload Options
Phosphate, an essential dietary micronutrient, is dysregulated in chronic kidney disease, and both cancer and periodontal disease are associated with chronic kidney disease. Reviewed evidence includes the association between phosphate toxicity and cancer development, and the association between periodontal disease and chronic kidney disease-mineral and bone disorder includes conditions such as ectopic calcification and bone resorption, which may be indirectly related to periodontal disease. Dental calculus in periodontal disease contains calcium phosphate crystals that are deposited from excess calcium and phosphate in saliva. Alveolar bone resorption may be linked systemically to release of parathyroid hormone in response to hypocalcemia induced by hyperphosphatemia.
1. Introduction
2. Phosphate Toxicity as a Global Health Burden
3. Dysregulated Phosphate Metabolism and Cancer
4. Periodontal Disease
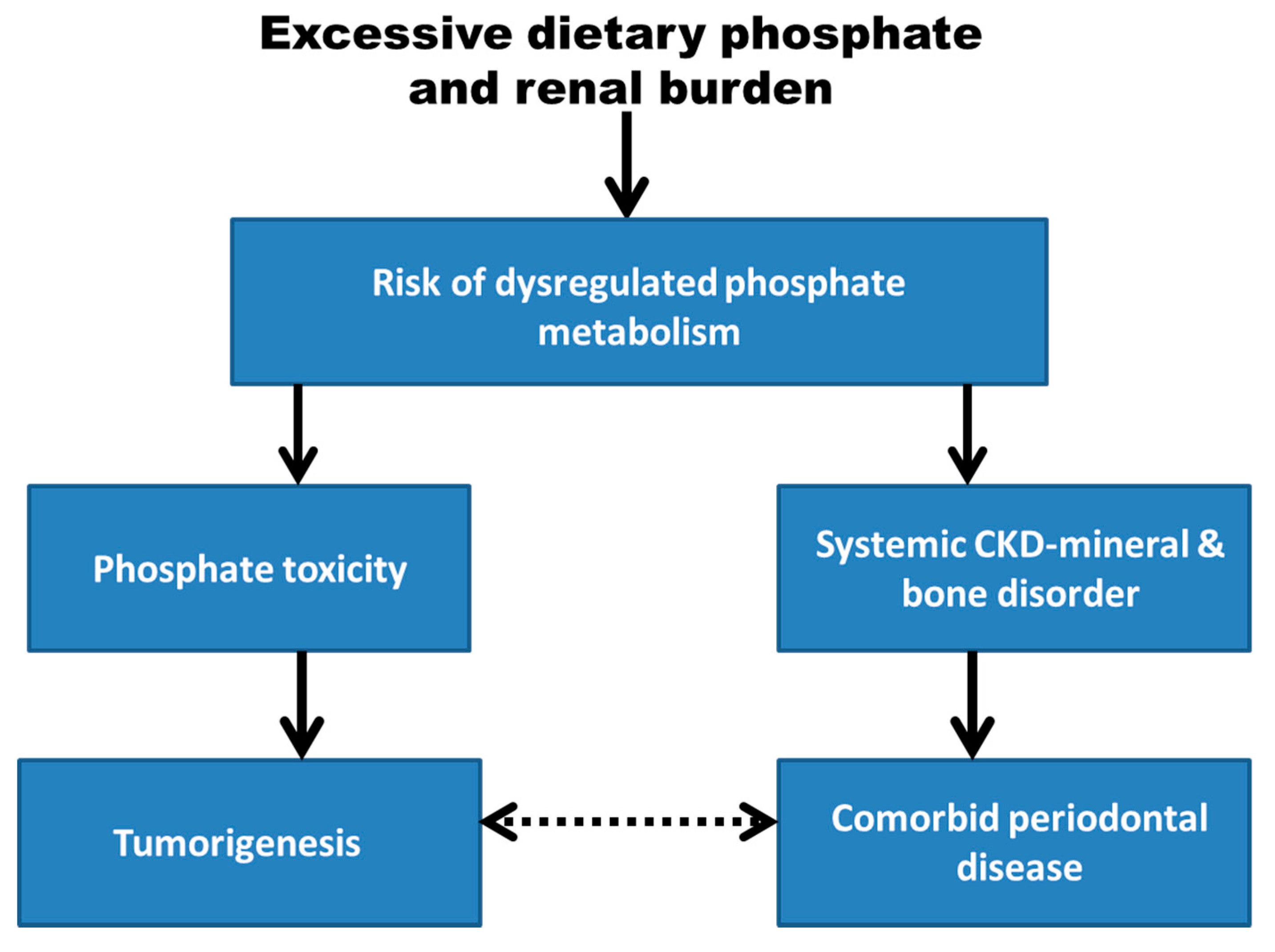
5. Chronic Kidney Disease-Mineral and Bone Disorder
References
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462.
- Corbella, S.; Veronesi, P.; Galimberti, V.; Weinstein, R.; Del Fabbro, M.; Francetti, L. Is periodontitis a risk indicator for cancer? A meta-analysis. PLoS ONE 2018, 13, e0195683.
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58.
- Mai, X.; LaMonte, M.J.; Hovey, K.M.; Freudenheim, J.L.; Andrews, C.A.; Genco, R.J.; Wactawski-Wende, J. Periodontal disease severity and cancer risk in postmenopausal women: The Buffalo OsteoPerio Study. Cancer Causes Control 2016, 27, 217–228.
- Nwizu, N.N.; Marshall, J.R.; Moysich, K.; Genco, R.J.; Hovey, K.M.; Mai, X.; LaMonte, M.J.; Freudenheim, J.L.; Wactawski-Wende, J. Periodontal Disease and Incident Cancer Risk among Postmenopausal Women: Results from the Women’s Health Initiative Observational Cohort. Cancer Epidemiol. Prev. Biomark. 2017, 26, 1255–1265.
- Michaud, D.; Kelsey, K.; Papathanasiou, E.; Genco, C.; Giovannucci, E. Periodontal disease and risk of all cancers among male never smokers: An updated analysis of the Health Professionals Follow-up Study. Ann. Oncol. 2016, 27, 941–947.
- Freudenheim, J.L.; Genco, R.J.; LaMonte, M.J.; Millen, A.E.; Hovey, K.M.; Mai, X.; Nwizu, N.; Andrews, C.A.; Wactawski-Wende, J. Periodontal disease and breast cancer: Prospective cohort study of postmenopausal women. Cancer Epidemiol. Prev. Biomark. 2016, 25, 43–50.
- Shi, T.; Min, M.; Sun, C.; Zhang, Y.; Liang, M.; Sun, Y. Periodontal disease and susceptibility to breast cancer: A meta-analysis of observational studies. J. Clin. Periodontol. 2018, 45, 1025–1033.
- Michaud, D.S.; Izard, J. Microbiota, oral microbiome, and pancreatic cancer. Cancer J. 2014, 20, 203.
- Maisonneuve, P.; Amar, S.; Lowenfels, A.B. Periodontal disease, edentulism, and pancreatic cancer: A meta-analysis. Ann. Oncol. 2017, 28, 985–995.
- Javed, F.; Warnakulasuriya, S. Is there a relationship between periodontal disease and oral cancer? A systematic review of currently available evidence. Crit. Rev. Oncol./Hematol. 2016, 97, 197–205.
- Yao, Q.-W.; Zhou, D.-S.; Peng, H.-J.; Ji, P.; Liu, D.-S. Association of periodontal disease with oral cancer: A meta-analysis. Tumor Biol. 2014, 35, 7073–7077.
- Momen-Heravi, F.; Babic, A.; Tworoger, S.S.; Zhang, L.; Wu, K.; Smith-Warner, S.A.; Ogino, S.; Chan, A.T.; Meyerhardt, J.; Giovannucci, E. Periodontal disease, tooth loss and colorectal cancer risk: Results from the Nurses’ Health Study. Int. J. Cancer 2017, 140, 646–652.
- Barton, M.K. Evidence accumulates indicating periodontal disease as a risk factor for colorectal cancer or lymphoma. CA: Cancer J. Clin. 2017, 67, 173–174.
- Bertrand, K.A.; Shingala, J.; Evens, A.; Birmann, B.M.; Giovannucci, E.; Michaud, D.S. Periodontal disease and risk of non-Hodgkin lymphoma in the Health Professionals Follow-Up Study. Int. J. Cancer 2017, 140, 1020–1026.
- Zeng, X.-T.; Deng, A.-P.; Li, C.; Xia, L.-Y.; Niu, Y.-M.; Leng, W.-D. Periodontal disease and risk of head and neck cancer: A meta-analysis of observational studies. PLoS ONE 2013, 8, e79017.
- Zeng, X.T.; Xia, L.Y.; Zhang, Y.G.; Li, S.; Leng, W.D.; Kwong, J.S. Periodontal Disease and Incident Lung Cancer Risk: A Meta-Analysis of Cohort Studies. J. Periodontol. 2016, 87, 1158–1164.
- Chrysanthakopoulos, N. Correlation between periodontal disease indices and lung cancer in Greek adults: A case—control study. Exp. Oncol. 2016, 38, 49–53.
- Lee, J.-H.; Kweon, H.H.-I.; Choi, J.-K.; Kim, Y.-T.; Choi, S.-H. Association between periodontal disease and prostate cancer: Results of a 12-year longitudinal cohort study in South Korea. J. Cancer 2017, 8, 2959.
- Chrysanthakopoulos, N.A.; Oikonomou, A.A. A case-control study of the periodontal condition in gastric cancer patients. Stomatol. Dis. Sci. 2017, 1, 55–61.
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity: A stealth biochemical stress factor? Med. Mol. Morphol. 2016, 49, 1–4.
- Erem, S.; Razzaque, M.S. Dietary phosphate toxicity: An emerging global health concern. Histochem. Cell Biol. 2018, 150, 711–719.
- Osuka, S.; Razzaque, M.S. Can features of phosphate toxicity appear in normophosphatemia? J. Bone Miner. Metab. 2012, 30, 10–18.
- Ohnishi, M.; Razzaque, M.S. Dietary and genetic evidence for phosphate toxicity accelerating mammalian aging. FASEB J. 2010, 24, 3562–3571.
- Razzaque, M.S. Phosphate toxicity: New insights into an old problem. Clin. Sci. 2011, 120, 91–97.
- Brown, R.B.; Razzaque, M.S. Phosphate toxicity and tumorigenesis. Biochimica et Biophysica Acta (BBA)-Rev. Cancer 2018, 1869, 303–309.
- Brown, R.B.; Razzaque, M.S. Dysregulation of phosphate metabolism and conditions associated with phosphate toxicity. Bonekey Rep. 2015, 4, 705.
- D’Arcangelo, M.; Brustugun, O.; Xiao, Y.; Choi, Y.; Behrens, C.; Solis, L.; Wang, Y.; Firestein, R.; Boyle, T.; Lund-Iversen, M. 194 Prevalence and prognostic significance of sodium-dependent phosphate transporter 2B (NaPi2B) protein expression in non-small cell lung cancer (NSCLC). Ann. Oncol. 2014, 25, iv66–iv67.
- Jacquemet, G.; Hamidi, H.; Ivaska, J. Filopodia in cell adhesion, 3D migration and cancer cell invasion. Curr. Opin. Cell Biol. 2015, 36, 23–31.
- Kuang, Y.; Nagy, J.D.; Elser, J.J. Biological stoichiometry of tumor dynamics: Mathematical models and analysis. Discret. Contin. Dyn. Syst. Ser. B 2004, 4, 221–240.
- Ward, D.; Griffin, A. Phosphorus incorporation into nucleic acids and proteins of liver nuclei of normal and azo dye-fed rats. Cancer Res. 1955, 15, 456–461.
- Wang, H.; Peng, R.; Wang, J.; Qin, Z.; Xue, L. Circulating microRNAs as potential cancer biomarkers: The advantage and disadvantage. Clin. Epigenetics 2018, 10, 59.
- Papaloucas, C.D.; Papaloucas, M.D.; Kouloulias, V.; Neanidis, K.; Pistevou-Gompaki, K.; Kouvaris, J.; Zygogianni, A.; Mystakidou, K.; Papaloucas, A.C. Measurement of blood phosphorus: A quick and inexpensive method for detection of the existence of cancer in the body. Too good to be true, or forgotten knowledge of the past? Med. Hypotheses 2014, 82, 24–25.
- Wilson, K.M.; Shui, I.M.; Mucci, L.A.; Giovannucci, E. Calcium and phosphorus intake and prostate cancer risk: A 24-y follow-up study. Am. J. Clin. Nutr. 2015, 101, 173–183.
- Camalier, C.E.; Young, M.R.; Bobe, G.; Perella, C.M.; Colburn, N.H.; Beck, G.R. Elevated phosphate activates N-ras and promotes cell transformation and skin tumorigenesis. Cancer Prev. Res. 2010, 3, 359–370.
- Jin, H.; Xu, C.-X.; Lim, H.-T.; Park, S.-J.; Shin, J.-Y.; Chung, Y.-S.; Park, S.-C.; Chang, S.-H.; Youn, H.-J.; Lee, K.-H. High dietary inorganic phosphate increases lung tumorigenesis and alters Akt signaling. Am. J. Respir. Crit. Care Med. 2009, 179, 59–68.
- Cheng, Z.; Hu, J.; King, J.; Jay, G.; Campbell, T. Inhibition of hepatocellular carcinoma development in hepatitis B virus transfected mice by low dietary casein. Hepatology 1997, 26, 1351–1354.
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951.
- Lin, Y.; McKinnon, K.E.; Ha, S.W.; Beck, G.R. Inorganic phosphate induces cancer cell mediated angiogenesis dependent on forkhead box protein C2 (FOXC2) regulated osteopontin expression. Mol. Carcinog. 2015, 54, 926–934.
- Liu, P.; Cheng, H.; Roberts, T.M.; Zhao, J.J. Targeting the phosphoinositide 3-kinase (PI3K) pathway in cancer, Nature reviews. Drug Discov. 2009, 8, 627.
- Chudek, J.; Nagy, A.; Kokot, F.; Podwinski, A.; Wiecek, A.; Ritz, E.; Kovacs, G. Phosphatemia is related to chromosomal aberrations of parathyroid glands in patients with hyperparathyroidism. J. Nephrol. 2007, 20, 164–172.
- Bobko, A.A.; Eubank, T.D.; Driesschaert, B.; Dhimitruka, I.; Evans, J.; Mohammad, R.; Tchekneva, E.E.; Dikov, M.M.; Khramtsov, V.V. Interstitial Inorganic Phosphate as a Tumor Microenvironment Marker for Tumor Progression. Sci. Rep. 2017, 7, 41233.
- Yamada, S.; Tokumoto, M.; Tatsumoto, N.; Taniguchi, M.; Noguchi, H.; Nakano, T.; Masutani, K.; Ooboshi, H.; Tsuruya, K.; Kitazono, T. Phosphate overload directly induces systemic inflammation and malnutrition as well as vascular calcification in uremia. Am. J. Physiol.-Renal Physiol. 2014, 306, F1418–F1428.
- NIDCR. Periodontal (Gum) Disease. 2018. Available online: https://www.nidcr.nih.gov/research/data-statistics/periodontal-disease (accessed on 28 December 2018).
- Pendyala, G.; Joshi, S.; Chaudhari, S.; Gandhage, D. Links demystified: Periodontitis and cancer. Dent. Res. J. 2013, 10, 704.
- Akcalı, A.; Lang, N.P. Dental calculus: The calcified biofilm and its role in disease development. Periodontology 2000 2018, 76, 109–115.
- Mendes, L.; Azevedo, N.F.; Felino, A.; Pinto, M.G. Relationship between invasion of the periodontium by periodontal pathogens and periodontal disease: A systematic review. Virulence 2015, 6, 208–215.
- Askar, A.M. Hyperphosphatemia: The hidden killer in chronic kidney disease. Saudi Med. J. 2015, 36, 13.
- Iff, S.; Craig, J.C.; Turner, R.; Chapman, J.R.; Wang, J.J.; Mitchell, P.; Wong, G. Reduced Estimated GFR and Cancer Mortality. Am. J. Kidney Dis. 2014, 63, 23–30.
- Linden, G.J.; Lyons, A.; Scannapieco, F.A. Periodontal systemic associations: Review of the evidence. J. Periodontol. 2013, 84, S8–S19.
- Grubbs, V.; Vittinghoff, E.; Taylor, G.; Kritz-Silverstein, D.; Powe, N.; Bibbins-Domingo, K.; Ishani, A.; Cummings, S.R. The association of periodontal disease with kidney function decline: A longitudinal retrospective analysis of the MrOS dental study. Nephrol. Dial. Transpl. 2015, 31, 466–472.
- Cholewa, M.; Madziarska, K.; Radwan-Oczko, M. The association between periodontal conditions, inflammation, nutritional status and calcium-phosphate metabolism disorders in hemodialysis patients. J. Appl. Oral Sci. 2018, 26, e20170495.
- Hou, Y.C.; Lu, C.L.; Lu, K.C. Mineral bone disorders in chronic kidney disease. Nephrology 2018, 23, 88–94.
- Kanjevac, T.; Bijelic, B.; Brajkovic, D.; Vasovic, M.; Stolic, R. Impact of Chronic Kidney Disease Mineral and Bone Disorder on Jaw and Alveolar Bone Metabolism: A Narrative Review. Oral Health Prev. Dent. 2018, 16, 79–85.
- Ausavarungnirun, R.; Wisetsin, S.; Rongkiettechakorn, N.; Chaichalermsak, S.; Udompol, U.; Rattanasompattikul, M. Association of dental and periodontal disease with chronic kidney disease in patients of a single, tertiary care centre in Thailand. BMJ Open 2016, 6, e011836.
- Zitt, E.; Lamina, C.; Sturm, G.; Knoll, F.; Lins, F.; Freistätter, O.; Kronenberg, F.; Lhotta, K.; Neyer, U. Interaction of time-varying albumin and phosphorus on mortality in incident dialysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2650–2656.
- Lieverse, A.R. Diet and the aetiology of dental calculus. Int. J. Osteoarchaeol. 1999, 9, 219–232.
- Tawfig, A. Dental Calculus Formation among Recurrent Renal Calculi Formers. Int. J. Dent. Oral Heal 2017, 3, 1–7.
- Lim, E.; Hyun, S.; Lee, J.M.; Kim, S.; Lee, M.-J.; Lee, S.-M.; Oh, Y.-S.; Park, I.; Shin, G.-T.; Kim, H. Effects of education on low-phosphate diet and phosphate binder intake to control serum phosphate among maintenance hemodialysis patients: A randomized controlled trial. Kidney Res. Clin. Pract. 2018, 37, 69.
- Martins, C.; Siqueira, W.L.; Oliveira, E.; Nicolau, J.; Primo, L.G. Dental calculus formation in children and adolescents undergoing hemodialysis. Pediatr. Nephrol. 2012, 27, 1961–1966.
- Fiyaz, M.; Ramesh, A.; Ramalingam, K.; Thomas, B.; Shetty, S.; Prakash, P. Association of salivary calcium, phosphate, pH and flow rate on oral health: A study on 90 subjects. J. Indian Soc. Periodontol. 2013, 17, 454.
- Panta, P.; Venna, V.R. Salivary RNA signatures in oral cancer detection. Anal. Cell. Pathol. 2014, 2014, 450629.
- Kamak, G.; Yildirim, E.; Rencber, E. Evaluation of the relationship between periodontal risk and carotid artery calcifications on panoramic radiographs. Eur. J. Dent. 2015, 9, 483.
- Sen, S.; Chung, M.; Duda, V.; Giamberardino, L.; Hinderliter, A.; Offenbacher, S. Periodontal disease associated with aortic arch atheroma in patients with stroke or transient ischemic attack. J. Stroke Cerebrovasc. Dis. 2017, 26, 2137–2144.




