Phosphate, an essential dietary micronutrient, is dysregulated in chronic kidney disease, and both cancer and periodontal disease are associated with chronic kidney disease. Reviewed evidence includes the association between phosphate toxicity and cancer development, and the association between periodontal disease and chronic kidney disease-mineral and bone disorder includes conditions such as ectopic calcification and bone resorption, which may be indirectly related to periodontal disease. Dental calculus in periodontal disease contains calcium phosphate crystals that are deposited from excess calcium and phosphate in saliva. Alveolar bone resorption may be linked systemically to release of parathyroid hormone in response to hypocalcemia induced by hyperphosphatemia.
- phosphate toxicity
- periodontal disease
- cancer
- tumorigenesis
- chronic kidney disease-mineral and bone disorder
- dental calculus
- resorption
- RNA
1. Introduction
2. Phosphate Toxicity as a Global Health Burden
Phosphorus, an essential dietary micronutrient, is ingested as phosphate in food and food additives. The highest amount of phosphate within the body is stored in bone as calcium phosphate. Levels of serum phosphate are regulated through a hormonal network, involving the kidneys, parathyroid glands, intestines, and the skeletal system [21]. Bioactive vitamin D, 1,25(OH)2D3, increases intestinal absorption of dietary phosphate, mainly through enhanced expression of sodium-phosphate 2b cotransporters. Fibroblast growth factor 23 (FGF23) is produced within osteocytes and osteoblasts of bone. Working in conjunction with its co-factor, Klotho, FGF23 lowers serum phosphate levels, and increases urinary phosphate excretion by suppressing reabsorption through action of sodium-phosphate cotransporters in the kidneys. Phosphorus renal reabsorption is also decreased by parathyroid hormone (PTH). If phosphate is dysregulated due to kidney burden and excessive dietary phosphate intake, serum phosphate levels may rise (hyperphosphatemia) and excessive phosphate may be sequestered in cellular tissue producing a pathological condition called phosphate toxicity. Phosphate toxicity is emerging as a global health concern as average amounts of dietary phosphate intake increase to approximately double the Recommended Dietary Allowance of 700 mg per day for an adult [22]. An excessive amount of phosphate stored in the body tissue may not always correlate with serum levels. It is possible that phosphate toxicity may be present in the body cells even in normophosphatemia, disturbing the function of almost every system in the body, including the muscular, skeletal, and vascular systems, and increasing morbidity and mortality [23]. Genetic evidence from animal experiments shows that phosphate toxicity may also accelerate mammalian aging [24], and phosphate toxicity is associated with tumorigenesis [25].3. Dysregulated Phosphate Metabolism and Cancer
Evidence supporting the role of dysregulated phosphate metabolism and phosphate toxicity in tumorigenesis has been detailed elsewhere [26][27][26,27]; a very brief summary of that evidence with important relevance to periodontal disease is presented here. Cancer cells express more phosphate cotransporters within their cell membranes than normal cells [28], which allow cancer cells to absorb and retain greater amounts of phosphate from the tumor microenvironment. Solid tumors have filopodia and lamellipodia that extend cancer cell membranes throughout the tumor microenvironment [29]. Phosphorus is a limiting factor in biological growth [30] and is the least abundantly supplied element in the formation of nucleic acids DNA and RNA. The sequestration of dysregulated amounts of phosphorus in cancer cells is associated with additional biosynthesis of ribosomal RNA [31], which increases protein synthesis necessary for cancer cell growth and tumorigenesis. Of relevance, detection of the overexpression of circulating microRNA fragments associated with dysregulated RNA biogenesis has potential as a cancer biomarker [32]. While serum phosphorus levels are not always a reliable indicator of phosphate stored in the body, a study of cancer patients found that they had abnormally higher serum phosphate levels compared to control patients [33]. The Health Professionals Follow-Up Study found that high-grade prostate cancer was associated with high dietary phosphate levels [34]. High dietary phosphorus fed to experimental animals caused skin cancer [35] and lung tumors [36]. Of relevance, experimental studies showing causative effects of cancer from feeding the milk protein casein [37] may not have controlled for high levels of phosphorus within casein, which is classified as a phosphoprotein [38]. Other studies have shown that high amounts of phosphate stimulate tumor neovascularization [39] and cell signaling in cancer growth [40], and are associated with chromosome instability [41] and metastasis [42]. Phosphate toxicity also contributes to systemic inflammation and malnutrition [43], which is seen in terminally ill cancer patients with cachexia.4. Periodontal Disease
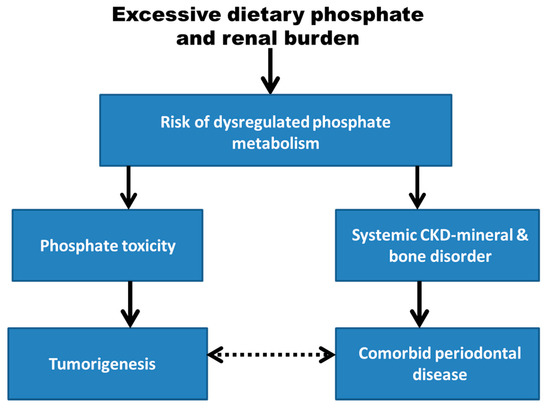
The periodontium functions to connect teeth to bone; its structures consist of the cementum, periodontal ligament, gingiva, and alveolar bone. Periodontal disease is the most common cause of adult tooth loss [44]. Many mediators of inflammation in periodontal disease are associated with cancer risk, such as C-reactive protein (CRP), Matrix metalloprotenases (MMP), Tumor Necrosis Factor (TNF), and Interluekin (IL) [45]. Inflammation within the periodontium may begin as gingivitis and eventually progress to periodontitis, which is usually associated with the accumulation of dental plaque or calculus. Dental calculus is formed supragingvally and subgingivally when biofilms rich in bacteria are mineralized with various crystals of calcium phosphate, including octacalcium phosphate = Ca4H(PO4)3 · 2H2O, brushite = CaH(PO4) · 2H2O, hydroxyapatite = Ca5(PO4)3(OH), and whitlockite that contains a small amount of magnesium and other elements = β-Ca3(PO4)2 [46]. While growth of bacteria in the oral microbiota is associated with periodontal disease, there is currently insufficient evidence to either support or exclude a causative role of bacterial invasion in the etiology of periodontal disease [47]. As an alternative explanation for the development of periodontal disease and its association with cancer, Figure 1 in this peresearchspective article shows that excessive dietary phosphate and renal burden may increase the risk for dysregulated phosphate metabolism. It is hypothesized that this systemic metabolic pathology could mediate the association of tumorigenesis with periodontal disease through separate causal pathways involving phosphate toxicity and systemic chronic kidney disease-mineral and bone disorder (CKD-MBD), respectively.