Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Filipa Mascarenhas-Melo | -- | 1824 | 2024-03-11 13:47:37 | | | |
| 2 | Wendy Huang | Meta information modification | 1824 | 2024-03-12 01:57:48 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ribeiro, M.; Simões, M.; Vitorino, C.; Mascarenhas-Melo, F. Applications of Hydrogels as Wound Dressings. Encyclopedia. Available online: https://encyclopedia.pub/entry/56115 (accessed on 03 March 2026).
Ribeiro M, Simões M, Vitorino C, Mascarenhas-Melo F. Applications of Hydrogels as Wound Dressings. Encyclopedia. Available at: https://encyclopedia.pub/entry/56115. Accessed March 03, 2026.
Ribeiro, Mariana, Marco Simões, Carla Vitorino, Filipa Mascarenhas-Melo. "Applications of Hydrogels as Wound Dressings" Encyclopedia, https://encyclopedia.pub/entry/56115 (accessed March 03, 2026).
Ribeiro, M., Simões, M., Vitorino, C., & Mascarenhas-Melo, F. (2024, March 11). Applications of Hydrogels as Wound Dressings. In Encyclopedia. https://encyclopedia.pub/entry/56115
Ribeiro, Mariana, et al. "Applications of Hydrogels as Wound Dressings." Encyclopedia. Web. 11 March, 2024.
Copy Citation
Hydrogels are polymeric materials that possess a set of characteristics meeting various requirements of an ideal wound dressing, making them promising for wound care. These features include, among others, the ability to absorb and retain large amounts of water and the capacity to closely mimic native structures, such as the extracellular matrix, facilitating various cellular processes like proliferation and differentiation. The polymers used in hydrogel formulations exhibit a broad spectrum of properties, allowing them to be classified into two main categories: natural polymers like collagen and chitosan, and synthetic polymers such as polyurethane and polyethylene glycol.
hydrogels
wound healing
natural polymers
synthetic polymers
cell adhesion
cell proliferation
biosensors
1. Introduction
The skin is the largest organ of the human body, accounting for almost 10% of the total body mass [1][2]. It serves as a fundamental anatomical barrier against pathogens and protects the external environment. The skin performs several important functions for maintaining the balance between the biological system and the surrounding environment, such as controlling the thermoregulation process. Furthermore, it is the human organ most frequently injured [1][2][3].
Thousands of years ago, ancient civilizations like the Greeks and Egyptians used tree bark, turmeric, aloe vera, and honey to treat wounds. The increased perception that injured skin is susceptible to contamination and dehydration boosted the development of both synthetic and natural dressings [4].
Since the 1960s, wound dressings have been considered favorable for wound healing because they create an environment conducive to skin regeneration [2]. The application of wound dressings aims to cover the wound, promote re-epithelialization, prevent mechanical trauma, and protect it from infections [5].
The ideal dressing should ensure a moist environment and have the capacity to absorb tissue exudate while allowing gaseous exchange, which is related to its porosity. It must protect the wound against microorganisms and stimulate tissue regeneration. Additionally, it should be rigid enough to allow for fixation on the wound, while remaining flexible and elastic to adapt to body movements. Moreover, it must be biocompatible and biodegradable, ensuring that its by-products are safe. The dressing should provide mechanical stability, and be widely available and cost-effective [1][2][4][5][6][7][8][9].
Due to their intrinsic properties, hydrogels fulfill various requirements for an ideal wound dressing [10]. They offer protection against microorganisms and new lesions [3]. Additionally, they can absorb large amounts of water, up to thousands of times their dry weight [5][11]. Therefore, the highly hydrated three-dimensional (3D) polymeric network allows for the maintenance of a high level of moisture in the wound bed [11]. Moreover, they adhere to the wound but are also easily removable. Their transparency facilitates visual inspection of the wound, and they are customizable and easily adapt to the contours of the wound, promoting autolytic debridement (removal of debris and necrotic tissues), and intrinsically stimulating healing through various mechanisms [5]. These mechanisms include promoting angiogenesis (formation and growth of blood vessels) in wounds with poor perfusion, modulating the immune cells within the wound, or enhancing the migration of keratinocytes and fibroblasts in wound healing [12][13][14][15]. Hydrogels overcome some limitations of traditional treatments, such as prolonged healing, limited body movement, traumatic removal, and poor regeneration of skin attachments [6].
Depending on the type of polymer that constitutes the hydrogel, it can be classified as natural or synthetic. Natural polymers offer better biocompatibility, while synthetic polymers exhibit improved mechanical strength and adjusted properties [3][9]. Regenerative medicine takes advantage of natural polymers, especially as dressings for wound treatment, due to their intrinsic characteristics of biocompatibility and biodegradability. They easily induce tissue repair and skin regeneration because of their interconnected 3D networks embedded in water or biological fluids, as well as their similarity to the extracellular matrix (ECM) [16].
Hydrogels are promising candidates for the treatment of cutaneous wounds and can be approached in different ways given the potential of their characteristics. Some of the applications of hydrogels as dressings include promoting wound healing through their inherent properties, delivering substances such as drugs and growth factors, enabling cell growth within their 3D structure, which mimics the native structures of the skin, or, more recently, incorporating biosensors for real-time monitoring of wound characteristics. Figure 1 provides a schematic representation of these applications.
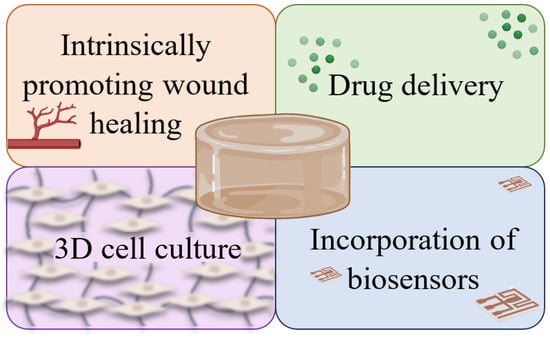
Figure 1. Representation of some of the applications of hydrogels as wound dressings: intrinsic capacity to stimulate healing; drug delivery systems or transporters of other substances; support for cell growth; real-time monitoring of the state of wounds through the incorporation of biosensors.
2. Hydrogels with the Intrinsic Ability to Promote Wound Healing
Hydrogels can promote wound healing through their intrinsic characteristics, notably by providing an appropriate cell-friendly microenvironment, stimulating angiogenesis, a key factor for tissue regeneration, or, for example, recruiting cells involved in the healing process [2][3][11]. For instance, Sun et al. [17] prepared a hydrogel based on dextran and PEG for the treatment of third-degree burns in mice. The results show that the hydrogel’s structure allows for the infiltration of neutrophils, which, in turn, facilitates the degradation of the hydrogel during the repair phase. This results in the recruitment of endothelial progenitors and angiogenic cells, stimulating rapid in vivo neovascularization after one week of treatment. Epithelial repair was observed within 14 days, with complete skin and appendage regeneration (sebaceous glands and hair follicles) noted by the end of 21 days. Another example is the hydrogel prepared by Yang et al. [18], composed of SF, HA, and alginate. SF serves as the primary matrix for tissue formation, providing mechanical support and promoting cell adhesion and proliferation. HA enhances biocompatibility, angiogenesis, and tissue regeneration, while alginate improves biocompatibility and hydrophilic performance. The hydrogel mimics the structure of the ECM in native tissue. This 3D porous microstructure possesses soft and elastic characteristics, along with good physical stability in environments simulating bodily fluids, ensuring adequate mechanical performance. These features enable the adhesion, growth, and proliferation of NIH-3T3 fibroblasts in vitro. In vivo, the hydrogel creates a favorable environment for healing, facilitating enhanced re-epithelialization, increased collagen deposition, ECM remodeling, and improved angiogenesis, thereby accelerating the burn wound healing process.
3. Hydrogels as Drug Delivery Systems and Other Substance Carriers
Furthermore, the matrix of hydrogels allows for the incorporation of substances, such as growth factors, cytokines, or drugs, namely, antibacterials and anti-inflammatories, that assist in the healing process [3][11]. Numerous studies focus on incorporating growth factors into hydrogels to expedite skin healing, such as β-FGF, primary promoters of cell proliferation with chemotactic activity, and VEGF, which enhances cell proliferation and tissue remodeling in wounds [4]. Similarly, in the study conducted by Hu et al. [19], they loaded EGF into a carboxymethyl chitosan and alginate hydrogel to protect the EGF from proteolytic degradation (ensuring its bioactivity) and to allow for its gradual release, improving cell proliferation. Dong et al. [20] prepared an SF hydrogel loaded with ciprofloxacin for the healing of deep partial-thickness burns in rats. The results showed that the hydrogel effectively delivered the antibiotic, inhibiting in vitro bacterial growth and biofilm formation of S. aureus and P. aeruginosa. In addition, the hydrogel promoted autolysis, a reduction in inflammation, fibroblast proliferation (attributed to the silk protein environment), increased collagen deposition, accelerated re-epithelialization, formation of granulation tissue, stimulation of angiogenesis, and showed complete reconstitution of the epidermal layer in rats after 18 days. In the study conducted by Yin et al. [21], they also prepared an SF-based hydrogel, but this time loaded it with rhein to simultaneously prevent bacterial colonization/infection and reduce inflammation. Rhein is a bioactive anthraquinone isolated from the traditional Chinese medicine rhubarb that possesses good anti-inflammatory and antibacterial properties. By incorporating it into SF hydrogels, the stability and structural integrity of rhein might be improved, thus enhancing therapeutic efficacy and minimizing negative effects. The SF/Rhein composite hydrogels combined the excellent biocompatibility and physicochemical properties of SF, along with the antibacterial and anti-inflammatory efficiency of rhein, accelerating the bacterially infected burn wound healing rate by reducing inflammation, expediting angiogenesis, and promoting skin appendages formation.
4. Hydrogels as 3D Scaffolds for Cell Adhesion and Proliferation
Hydrogels can serve as a platform for loading cells [3][11]. The 3D matrix of hydrogels allows for the deposition and organization of cells [8], mimicking the environment of the natural biological ECM better than two-dimensional substrates [22][23][24]. The architecture of the hydrogel, characterized by suitable biocompatibility, morphology, and mechanical behavior, enables it to function as a temporary support, facilitating cellular processes such as adhesion, proliferation, and differentiation for the formation of new tissue [25]. The hydrogel can be loaded with cells such as keratinocytes and fibroblasts, as demonstrated in the study by Mohamad et al. [8], where they loaded keratinocytes and fibroblasts into a hydrogel based on BC and acrylic acid. The results were very promising, achieving complete re-epithelialization of a partial-thickness burn within 13 days. Furthermore, there was a more organized deposition of type I collagen fibers, attributed to the synergistic effect of the hydrogel with the incorporated cells in accelerating skin regeneration and strengthening the dermis. Additionally, the incorporated cells can be stem cells. It is considered that stem cells promote healing through differentiation into specific cell types or through paracrine effects to stimulate the host tissue regeneration [26]. Stem cell therapies have shown promising outcomes in the context of wound healing [27][28]. Dong et al. [26] demonstrated that the HA and poly (ethylene glycol) hydrogel loaded with adipose tissue-derived stem cells provided an optimized 3D microenvironment, enhancing the therapeutic efficiency of stem cell-based therapies. The hydrogel promoted cell paracrine secretion and increased the expression of pro-angiogenic growth factors and cytokines, such as angiopoietin, VEGF, platelet-derived growth factor, stromal cell-derived factor, contributing to the wound healing treatment in a burn animal model.
5. Hydrogels with Integrated Biosensors
Moreover, the incorporation of biosensors into hydrogels can solve various challenges associated with wounds, such as the early detection of infections and the acquisition of relevant information about the wound microenvironment in real-time. This information can be used to provide timely and accurate reports on the evolution of the healing process [9][11]. Villanueva et al. [29] developed a smart antimicrobial wound dressing based on keratin hydrogels with zinc oxide nanoparticles (nZnO), taking advantage of the pH-responsive behavior of keratin and the antimicrobial activity of nZnO. Infected wounds exhibit alkaline pH due to the by-products of bacterial metabolism. As the wound undergoes healing, the pH becomes acidic. In a clean wound, the dressing acts as a barrier, isolating the injury from the external environment and protecting it from microbial contamination. In a bacterially contaminated wound, the increased pH leads to hydrogel swelling, increasing its pore size, and facilitating the release of the antimicrobial agent into the medium, thereby controlling the infection. Mostafalu et al. [30] developed an alginate hydrogel sheet incorporated with poly (N-isopropyl acrylamide) stimuli-responsive particles (a drug-releasing system), loaded with cefazolin for real-time monitoring of the wound environment for individualized treatment of chronic wounds. This automated, smart, flexible wound dressing comprises pH sensors and a microheater to trigger thermo-responsive drug carriers containing antibiotics. The pH sensors are connected to a microcontroller through an electronic module that processes the data measured by the sensors. Once the pH exceeds an acceptable range, it communicates wirelessly in a closed-loop manner to smartphones or computers to remotely activate the heater and program the on-demand release of the antibacterial drug.
References
- Varaprasad, K.; Jayaramudu, T.; Kanikireddy, V.; Toro, C.; Sadiku, E.R. Alginate-based composite materials for wound dressing application:A mini review. Carbohydr. Polym. 2020, 236, 116025.
- Zhang, L.; Yin, H.; Lei, X.; Lau, J.N.Y.; Yuan, M.; Wang, X.; Zhang, F.; Zhou, F.; Qi, S.; Shu, B.; et al. A Systematic Review and Meta-Analysis of Clinical Effectiveness and Safety of Hydrogel Dressings in the Management of Skin Wounds. Front. Bioeng. Biotechnol. 2019, 7, 342.
- Surowiecka, A.; Strużyna, J.; Winiarska, A.; Korzeniowski, T. Hydrogels in Burn Wound Management—A Review. Gels 2022, 8, 122.
- Sharma, S.; Rai, V.K.; Narang, R.K.; Markandeywar, T.S. Collagen-based formulations for wound healing: A literature review. Life Sci. 2022, 290, 120096.
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461.
- Fayyazbakhsh, F.; Khayat, M.J.; Leu, M.C. 3D-Printed Gelatin-Alginate Hydrogel Dressings for Burn Wound Healing: A Comprehensive Study. Int. J. Bioprint. 2022, 8, 274–291.
- Longinotti, C. The use of hyaluronic acid based dressings to treat burns: A review. Burn. Trauma 2014, 2, 162–168.
- Mohamad, N.; Loh, E.Y.X.; Fauzi, M.B.; Ng, M.H.; Mohd Amin, M.C.I. In vivo evaluation of bacterial cellulose/acrylic acid wound dressing hydrogel containing keratinocytes and fibroblasts for burn wounds. Drug Deliv. Transl. Res. 2019, 9, 444–452.
- Naseri, E.; Ahmadi, A. A review on wound dressings: Antimicrobial agents, biomaterials, fabrication techniques, and stimuli-responsive drug release. Eur. Polym. J. 2022, 173, 111293.
- Stubbe, B.; Mignon, A.; Declercq, H.; Van Vlierberghe, S.; Dubruel, P. Development of Gelatin-Alginate Hydrogels for Burn Wound Treatment. Macromol. Biosci. 2019, 19, 1900123.
- Tavakoli, S.; Klar, A.S. Advanced Hydrogels as Wound Dressings. Biomolecules 2020, 10, 1169.
- He, Y.; Cen, Y.; Tian, M. Immunomodulatory hydrogels for skin wound healing: Cellular targets and design strategy. J. Mater. Chem. B 2024, 12, 2435–2458.
- Kharaziha, M.; Baidya, A.; Annabi, N. Rational Design of Immunomodulatory Hydrogels for Chronic Wound Healing. Adv. Mater. 2021, 33, e2100176.
- Fan, F.; Saha, S.; Hanjaya-Putra, D. Biomimetic Hydrogels to Promote Wound Healing. Front. Bioeng. Biotechnol. 2021, 9, 718377.
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127.
- Mogoşanu, G.D.; Grumezescu, A.M. Natural and synthetic polymers for wounds and burns dressing. Int. J. Pharm. 2014, 463, 127–136.
- Sun, G.; Zhang, X.; Shen, Y.-I.; Sebastian, R.; Dickinson, L.E.; Fox-Talbot, K.; Reinblatt, M.; Steenbergen, C.; Harmon, J.W.; Gerecht, S. Dextran hydrogel scaffolds enhance angiogenic responses and promote complete skin regeneration during burn wound healing. Proc. Natl. Acad. Sci. USA 2011, 108, 20976–20981.
- Yang, W.; Xu, H.; Lan, Y.; Zhu, Q.; Liu, Y.; Huang, S.; Shi, S.; Hancharou, A.; Tang, B.; Guo, R. Preparation and characterisation of a novel silk fibroin/hyaluronic acid/sodium alginate scaffold for skin repair. Int. J. Biol. Macromol. 2019, 130, 58–67.
- You, S.; Huang, Y.; Mao, R.; Xiang, Y.; Cai, E.; Chen, Y.; Shen, J.; Dong, W.; Qi, X. Together is better: Poly(tannic acid) nanorods functionalized polysaccharide hydrogels for diabetic wound healing. Ind. Crop. Prod. 2022, 186, 115273.
- Dong, M.; Mao, Y.; Zhao, Z.; Zhang, J.; Zhu, L.; Chen, L.; Cao, L. Novel fabrication of antibiotic containing multifunctional silk fibroin injectable hydrogel dressing to enhance bactericidal action and wound healing efficiency on burn wound: In vitro and in vivo evaluations. Int. Wound J. 2022, 19, 679–691.
- Yin, C.; Han, X.; Lu, Q.; Qi, X.; Guo, C.; Wu, X. Rhein incorporated silk fibroin hydrogels with antibacterial and anti-inflammatory efficacy to promote healing of bacteria-infected burn wounds. Int. J. Biol. Macromol. 2022, 201, 14–19.
- Hu, X.; Xia, Z.; Cai, K. Recent advances in 3D hydrogel culture systems for mesenchymal stem cell-based therapy and cell behavior regulation. J. Mater. Chem. B 2022, 10, 1486–1507.
- Morales, X.; Cortés-Domínguez, I.; Ortiz-De-Solorzano, C. Modeling the Mechanobiology of Cancer Cell Migration Using 3D Biomimetic Hydrogels. Gels 2021, 7, 17.
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200.
- Hernández-Rangel, A.; Martin-Martinez, E.S. Collagen based electrospun materials for skin wounds treatment. J. Biomed. Mater. Res. Part A 2021, 109, 1751–1764.
- Dong, Y.; Cui, M.; Qu, J.; Wang, X.; Kwon, S.H.; Barrera, J.; Elvassore, N.; Gurtner, G.C. Conformable hyaluronic acid hydrogel delivers adipose-derived stem cells and promotes regeneration of burn injury. Acta Biomater. 2020, 108, 56–66.
- Sharma, P.; Kumar, A.; Dey, A.D.; Behl, T.; Chadha, S. Stem cells and growth factors-based delivery approaches for chronic wound repair and regeneration: A promise to heal from within. Life Sci. 2021, 268, 118932.
- Wang, M.; Xu, X.; Lei, X.; Tan, J.; Xie, H. Mesenchymal stem cell-based therapy for burn wound healing. Burn. Trauma 2021, 9, tkab002.
- Villanueva, M.E.; Cuestas, M.L.; Pérez, C.J.; Campo Dall’ Orto, V.; Copello, G.J. Smart release of antimicrobial ZnO nanoplates from a pH-responsive keratin hydrogel. J. Colloid Interface Sci. 2019, 536, 372–380.
- Mostafalu, P.; Tamayol, A.; Rahimi, R.; Ochoa, M.; Khalilpour, A.; Kiaee, G.; Yazdi, I.K.; Bagherifard, S.; Dokmeci, M.R.; Ziaie, B.; et al. Smart Bandage for Monitoring and Treatment of Chronic Wounds. Small 2018, 14, e1703509.
More
Information
Subjects:
Polymer Science
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
892
Revisions:
2 times
(View History)
Update Date:
12 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





