Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Filipa Mascarenhas-Melo and Version 2 by Wendy Huang.
Hydrogels are polymeric materials that possess a set of characteristics meeting various requirements of an ideal wound dressing, making them promising for wound care. These features include, among others, the ability to absorb and retain large amounts of water and the capacity to closely mimic native structures, such as the extracellular matrix, facilitating various cellular processes like proliferation and differentiation. The polymers used in hydrogel formulations exhibit a broad spectrum of properties, allowing them to be classified into two main categories: natural polymers like collagen and chitosan, and synthetic polymers such as polyurethane and polyethylene glycol.
- hydrogels
- wound healing
- natural polymers
- synthetic polymers
- cell adhesion
- cell proliferation
- biosensors
1. Introduction
The skin is the largest organ of the human body, accounting for almost 10% of the total body mass [1][2][1,2]. It serves as a fundamental anatomical barrier against pathogens and protects the external environment. The skin performs several important functions for maintaining the balance between the biological system and the surrounding environment, such as controlling the thermoregulation process. Furthermore, it is the human organ most frequently injured [1][2][3][1,2,3].
Thousands of years ago, ancient civilizations like the Greeks and Egyptians used tree bark, turmeric, aloe vera, and honey to treat wounds. The increased perception that injured skin is susceptible to contamination and dehydration boosted the development of both synthetic and natural dressings [4].
Since the 1960s, wound dressings have been considered favorable for wound healing because they create an environment conducive to skin regeneration [2]. The application of wound dressings aims to cover the wound, promote re-epithelialization, prevent mechanical trauma, and protect it from infections [5].
The ideal dressing should ensure a moist environment and have the capacity to absorb tissue exudate while allowing gaseous exchange, which is related to its porosity. It must protect the wound against microorganisms and stimulate tissue regeneration. Additionally, it should be rigid enough to allow for fixation on the wound, while remaining flexible and elastic to adapt to body movements. Moreover, it must be biocompatible and biodegradable, ensuring that its by-products are safe. The dressing should provide mechanical stability, and be widely available and cost-effective [1][2][4][5][6][7][8][9][1,2,4,5,6,7,8,9].
Due to their intrinsic properties, hydrogels fulfill various requirements for an ideal wound dressing [10]. They offer protection against microorganisms and new lesions [3]. Additionally, they can absorb large amounts of water, up to thousands of times their dry weight [5][11][5,11]. Therefore, the highly hydrated three-dimensional (3D) polymeric network allows for the maintenance of a high level of moisture in the wound bed [11]. Moreover, they adhere to the wound but are also easily removable. Their transparency facilitates visual inspection of the wound, and they are customizable and easily adapt to the contours of the wound, promoting autolytic debridement (removal of debris and necrotic tissues), and intrinsically stimulating healing through various mechanisms [5]. These mechanisms include promoting angiogenesis (formation and growth of blood vessels) in wounds with poor perfusion, modulating the immune cells within the wound, or enhancing the migration of keratinocytes and fibroblasts in wound healing [12][13][14][15][12,13,14,15]. Hydrogels overcome some limitations of traditional treatments, such as prolonged healing, limited body movement, traumatic removal, and poor regeneration of skin attachments [6].
Depending on the type of polymer that constitutes the hydrogel, it can be classified as natural or synthetic. Natural polymers offer better biocompatibility, while synthetic polymers exhibit improved mechanical strength and adjusted properties [3][9][3,9]. Regenerative medicine takes advantage of natural polymers, especially as dressings for wound treatment, due to their intrinsic characteristics of biocompatibility and biodegradability. They easily induce tissue repair and skin regeneration because of their interconnected 3D networks embedded in water or biological fluids, as well as their similarity to the extracellular matrix (ECM) [16].
Hydrogels are promising candidates for the treatment of cutaneous wounds and can be approached in different ways given the potential of their characteristics. Some of the applications of hydrogels as dressings include promoting wound healing through their inherent properties, delivering substances such as drugs and growth factors, enabling cell growth within their 3D structure, which mimics the native structures of the skin, or, more recently, incorporating biosensors for real-time monitoring of wound characteristics. Figure 1 provides a schematic representation of these applications.
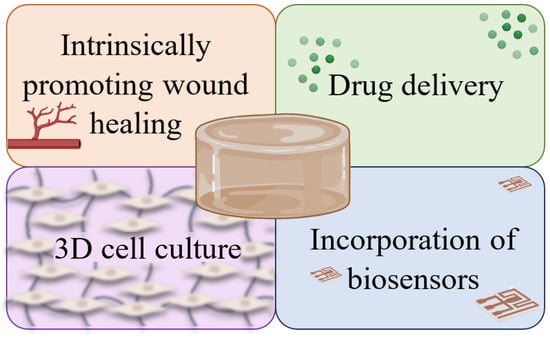

Figure 1. Representation of some of the applications of hydrogels as wound dressings: intrinsic capacity to stimulate healing; drug delivery systems or transporters of other substances; support for cell growth; real-time monitoring of the state of wounds through the incorporation of biosensors.
