
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carmen Zaharia | -- | 4046 | 2024-03-11 12:34:30 | | | |
| 2 | Dean Liu | Meta information modification | 4046 | 2024-03-12 01:47:06 | | | | |
| 3 | Dean Liu | + 1 word(s) | 4047 | 2024-04-15 07:14:55 | | |
Video Upload Options
The permanent demand of modern society for water consumption across different industrial and domestic activities involves an increasing requirement for effective facilities that can ensure the treatment of the produced WW (Wastewater) for onsite reuse, recycling, and safe/non-polluting discharge of the final effluents to natural aquatic environments. A few fundamental aspects of WW treatment using different physical, chemical, and biological processes were discussed, with the central goal being focused on the coagulation–flocculation step. Therefore, the role of the coagulation–flocculation step when applied to the treatment of colored textile WW and the advantages and disadvantages of using different chemicals as coagulation–flocculation agents in some industrial WW treatment systems as well as hybrid materials were presented in association with their increased efficiency in comparison to conventional ones.
1. Textile WW Treatment Processes for Dye(s) and Color Removal
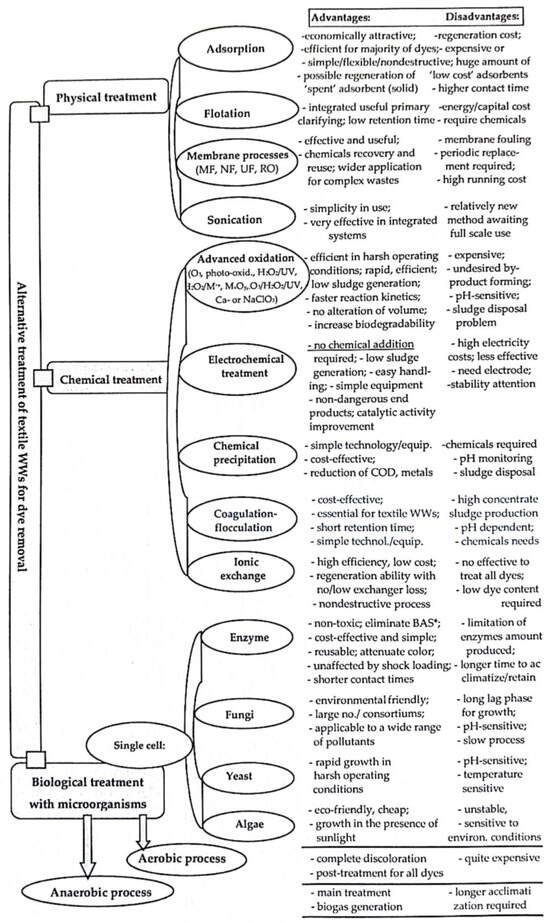
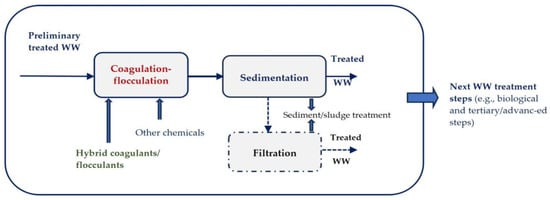
2. Coagulation–Flocculation Technologies and Its Performances in Colored Textile WWs Treatment
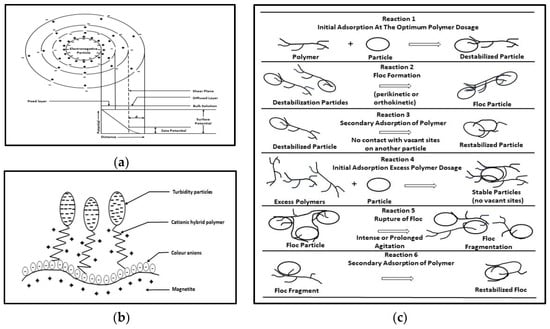
- –
-
Attraction forces: these decrease the surface charge and enhance the aggregation of solids in distinct, separable aggregates or flocs (coagulation process).
- –
-
The simple electrostatic adsorption of counterions: effectively neutralizes the solid surface charge and decreases the surface potential (dependent on ionic species or large, complex molecules, and ordinary adsorption) (coagulation–flocculation process).
- –
-
Precipitation: hydrated metal hydroxides (precipitates) are formed that can adsorb on the solid surface with other existing colloids and neutralize the surface charge (pH-sensitive, with the characteristic value of the isoelectric point of metal hydroxide) (coagulation–flocculation process).
- –
-
Enmeshment in an agglomerate precipitate and adsorption: is when organic polymers are used (cationic, anionic, and nonionic ones). The existing ions in the WW interact with the polymeric chains, forming solid aggregates (flocculation process).
References
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Proc. Safety Environ. Prot. 2020, 143, 136–163.
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluents: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255.
- Zaharia, C.; Suteu, D. Textile Organic Dyes—Characteristics, Polluting effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants—Ten Years after the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech: Rijeka, Croatia, 2012; Chapter 3; pp. 55–86. ISBN 978-953-307-917-2.
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164.
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505.
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84.
- Ramos, M.D.N.; Pereira Lima, J.P.; de Aquino, S.F.; Aguiar, A. A critical analysis of the alternative treatments applied to effluents from Brazilian textile industries. J. Water Proc. Eng. 2021, 43, 102273.
- Macoveanu, M.; Teodosiu, C.; Duca, G. Advanced Treatment of Wastewaters with Non-Biodegradable Organic Compounds; Technical University Ed.; Gheorghe Asachi: Iasi, Romania, 1997. (In Romanian)
- Zaharia, C. Innovative Wastewater Treatment Technologies: Opportunities, Perspectives and Challenges; Ecozone Ed.: Iasi, Romania, 2023; ISBN 978-606-8625-39-3.
- Musteret, C.P.; Fighir, D.; Gavrilescu, D.; Zaharia, C.; Teodosiu, C. Waters and Wastewaters Treatment: Practice Applications; Politehnium: Iasi, Romania, 2014; ISBN 978-973-621-442-4. (In Romanian)
- Cooper, P. Colour in Dyehouse Effluent; Publishing House of the Society of Dyers and Colourists: Nottingham, UK, 1995.
- Zaharia, C.; Suteu, D.; Muresan, A. Options and solutions for textile effluent decolorization using some specific physico-chemical treatment steps. Environ. Eng. Manag. J. 2012, 11, 493–509.
- Zaharia, C.; Surpateanu, M. Study of flocculation with Prodefloc CRC 301 polyelectrolyte applied into a chemical wastewater treatment. Ovidius Univ. Annals Chem. 2006, 17, 50–53.
- Zaharia, C.; Diaconescu, R.; Surpateanu, M. Optimization study of a wastewater chemical treatment with PONILIT GT-2 anionic polyelectrolyte. Environ. Eng. Manag. J. 2006, 5, 1141–1152.
- Zaharia, C.; Diaconescu, R.; Surpateanu, M. Study of flocculation with Ponilit GT-2 anionic polyelectrolyte applied into a chemical wastewater treatment. Open Chem. 2007, 5, 239–256.
- Khan, M.S.; Knapp, J.; Clemett, A.; Chadwick, M.; Mahmood, M.; Sharif, M.I. Managing and monitoring effluent treatment plants. In Managing Industrial Pollution from Small and Medium Scale Industries in Bangladesh; Booklet Series SEI, BCAS; University of Leeds: Leeds, UK, 2006.
- Crini, G.; Badot, P.M. Traitement et Épuration des Eaux Industrielles Polluées; PUFC: Besançon, France, 2010.
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of industrial effluents: Available methods and emerging technologies—A review. Rev. Environ. Sci. Bio/Technol. 2005, 4, 245–273.
- Berefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice-Hall: Saddle River, NJ, USA, 1982.
- Abujazar, M.S.; Karaagac, S.U.; Amr, S.S.A.; Alazaiza, M.Y.D.; Bashir, M.J.K. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133.
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697.
- Vajnhandl, S.; Valh, J.V. The status of water reuse in European textile sector. J. Environ. Manag. 2014, 141, 29–35.
- Bilinska, L.; Cmurek, M.; Ledakowicz, S. Textile wastewater treatment by AOPs for brine reuse. Process Saf. Environ. Prot. 2017, 109, 420–428.
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017.
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641.
- Fernandes, A.; Makos, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulphate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean Prod. 2018, 195, 374–384.
- Nguyen, C.H.; Tran, M.I.; Van Tran, T.T.; Juang, R.S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysts over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962.
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The role of ultrasound on advanced oxidation processes. Top. Curr. Chem. 2016, 374, 75.
- Butani, S.A.; Mane, S.J. Coagulation/flocculation process for cationic, anionic dye removal using water treatment residuals—A review. Int. J. Technol. Manag. 2017, 6, 121–125.
- Collivignarelli, M.C.; Abba, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of art. J. Environ. Managn. 2019, 236, 727–745.
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Global NEST J. 2013, 15, 522–528.
- Chellam, S.; Sari, M.A. Aluminium electrocoagulation as pretreatment during microfiltration of surface water containing NOM: A review of fouling, NOM, DBP and virus content. J. Hazard. Mater. 2016, 304, 490–501.
- He, C.C.; Hu, C.Y.; Lo, S.L. Evaluation of sono-electrocoagulation for the removal of reactive Blue 19 passive film removed by ultrasound. Sep. Purif. Technol. 2016, 165, 107–113.
- Hamad, H.; Bassyouni, D.; El-Ashroukhy, E.S.; Amin, N.; El-Latif, M.A. Comparative performance of anode oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187.
- Rosales, E.; Pazos, M.; Longo, M.A.; Sanroman, M.A. Electro-Fenton decoloration of dyes in a continuous reactor: A promising technology in colored wastewater treatment. Chem. Eng. J. 2009, 155, 62–67.
- Dos Santos, A.J.; Garcia-Segura, S.; Dosta, S.; Cano, I.G.; Martinez-Huitie, C.A.; Brillas, E. A ceramic electrode of ZnO2-V2O5 for the generation of oxidant species in anodic oxidation. Assessment of the treatment of Acid Blue 29 dye in sulphate and chloride media. Sep. Purif. Technol. 2019, 228, 115747.
- Martinez-Huite, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71.
- Rambabu, K.; Bharach, C.; Monash, P.; Veiu, S.; Banat, F.; Naushat, M.; Arthanameswaran, G.; Show, P.I. Effective treatment of dye polluted wastewater using nanoporous CaCl2 modified polyethersulphone membrane. Process Saf. Environ. Prot. 2019, 124, 266–278.
- Gunawan, F.M.; Mangindzan, D.; Khoiruddin, K.; Wenten, I.C. Nanofiltration membrane cross-linked by m-phenylenediamine for dye removal from textile wastewater. Polym. Adv. Technol. 2019, 30, 360–367.
- Gao, J.; Thang, Z.; Wang, K.Y.; Cheng, T.S. Fabrication of loose inner-selective polyethersulphine (PES) hollow fibers by one-step spinning process for nanofiltration (NF) of textile dyes. J. Memb. Sci. 2017, 541, 413–424.
- Wiesmann, U.; Choi, I.S.; Dombrowski, E.M. Fundamentals of Biological Wastewater Treatment; Wiley-VCH Verlag GmbH&Co. KgaA: Weinheim, Germany, 2017.
- Ødegaard, H. Particle separation in wastewater treatment. In Proceedings of the 7th European Sewage and Refuse Symposium, Munich, Germany, 19–22 May 1987; pp. 351–400.
- Walldal, C. Electrokinetic study of silica particles flocculated by two cationic polyelectrolytes: Sequential and simultaneous addition. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 111–121.
- Yen, T.F. Chemical Processes for Environmental Engineering; Imperial College Press: Danvers, MA, USA, 2007; ISBN 978-1-86094-759-9.
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155.
- Uysal, Y.; Aktas, D.; Caglar, Y. Determination of colour removal efficiency of Lemna minor L. from industrial effluents. J. Environ. Prot. Ecol. 2014, 15, 1718–1726.
- Un, U.T.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and COD from real textile wastewater. J. Environ. Manag. 2013, 123, 113–119.
- Verma, A.K.; Bhunia, P.; Dash, R.R. Carbonaceous organics removal kinetics in an upflow anaerobic blanket (UASB) reactor treating physico-chemically pre-treated textile wastewater. Desal. Water Treat. 2015, 54, 1577–1588.
- Zaharia, C. Wastewater Chemical Treatment; Performantica: Iasi, Romania, 2006. (In Romanian)
- Haller, E.J. Simplified Wastewater Treatment Plant Operations; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1995; pp. 107–119.
- Qian, F.; Sun, X.; Liu, Y. Removal characteristics of organics in bio-treated textile wastewater reclamation by a stepwise coagulation and intermediate GAC/O3 oxidation process. Chem. Eng. J. 2013, 214, 112–118.
- Wang, J.; Guo, M.; Luo, Y.; Shao, D.; Ge, S.; Cai, L.; Xia, C.; Lan, S.S. Production of magnetic sodium alginate polyelectrolyte nanospheres for lead ions removal from wastewater. J. Environ. Manag. 2021, 289, 112506.
- Ukiwe, L.N.; Alinnor, J.I. Assessment of polyacrylamide and aluminium sulphate coagulants in turbidity removal in wastewater. Terr. Aquat. Environ. Toxicol. 2012, 6, 132–135.
- Kurniawan, S.B.; Imron, M.F.; Chik, C.E.N.C.E.; Owodunni, A.A.; Ahmad, A.; Alnawajha, M.M.; Rahim, N.F.M.; Said, N.S.M.; Sheikh Abdullah, S.R.; Kasan, N.A.; et al. What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci. Total Environ. 2022, 806, 150902.
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping polyelectrolyte composites for heavy metals adsorption from wastewater: Experimental assessment and equilibrium studies. J. Environ. Manag. 2022, 321, 115999.
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid materials for the removal of emerging pollutants in water: Classification. Sysnthesis, and properties. Chem. Eng. J. Adv. 2022, 10, 100252.
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689.
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, characterization and the application of hybrid materials in coagulation/flocculation in wastewater: A review. Chem. Eng. J. 2012, 203, 370–386.
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 2022, 8, 100158.
- El-Taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Ahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358.
- Shi, C.; Wang, Q.; Li, D.; Zeng, B.; Liu, Q.; Cui, Y.; Wang, J.; Wang, X. Inorganic composite coagulant for wool scouring wastewater treatment: Performance, kinetics and coagulation mechanism. Sep. Purif. Technol. 2023, 313, 123482.
- Qadeer, H.A.; Mahomoodally, M.F.; Nadeem, F.; Khanam, A. Wastewater treatment and dyes removal using electrocoagulation aided by natural biosorbents—A review. Int. J. Chem. Biochem. Sci. 2018, 14, 77–87.
- Saravanan, A.; Thamarai, P.; Kumar, P.S.; Rangasamy, G. Recent advances in polymer composite, extraction, and their application for wastewater treatment: A review. Chemosphere 2022, 308, 136368.
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. J. Water Proc. Eng. 2021, 42, 102096.
- Bediako, J.K.; El Ouardi, Y.; Masima Mouele, E.S.; Mensah, B.; Repo, E. Polyelectrolyte and polyelectrolyte complex-incorporated adsorbents in water and wastewater remediation—A review of recent advances. Chemosphere 2023, 325, 138418.
- Elgarahy, A.M.; Maged, A.; Eloffy, M.G.; Zahran, M.; Kharbish, S.; Elwakeel, K.Z.; Bhatnagar, A. Geopolymers as sustainable eco-friendly materials: Classification, synthesis routes, and applications in wastewater treatment. Sep. Purif. Technol. 2023, 324, 124631.
- Nageswara, R.L.; Feroz, S.; Karunya, S.; Motilal, L.; Saidireddy, P.; Suman, G. Synthesis, characterization and application of polymer composite materials in wastewater treatment. Mater. Today Proc. 2022, 59, 1726–1734.
- Dutt, M.A.; Hanif, M.A.; Nadeem, F.; Bhatti, H.N. A review of advances in engineered composite materials popular for wastewater treatment. J. Env. Chem. Eng. 2020, 8, 104073.
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788.
- Lin, S.H.; Chen, M.L. Purification of textile wastewater effluents by a combined Fenton process and ion exchange. Desalination 1997, 109, 121–130.
- Venkat Mohan, S.; Srimurli, M.; Sailaja, P.; Karthikeyan, J. A study of acid dye colour removal using adsorption and coagulation. Environ. Eng. Policy 1999, 1, 149–154.
- Sanghi, R.; Bhattacharya, B.; Singh, V. Use of Cassia javahikai seed gumand gum polyacrylamide as coagulant aid for the decolorization of textile dye solutions. Biores. Technol. 2006, 97, 1259–1264.
- Chitanu, G.C.; Carpov, A.; Asaftei, T. Romanian Invention Patent (OSIM) No. 106745; ‘P. Poni’ Institute of Macromolecular Chemistry of Iasi: Iasi, Romania, 1987.
- Zaharia, C.; Macoveanu, M. Separation of some heavy metal ions from wastewaters using polyelectrolytes. Sci. Ann. 2000, VIII, 199–206.
- Zaharia, C.; Surpăţeanu, M.; Creţescu, I.; Macoveanu, M.; Braunstein, H. Electrocoagulation/electroflotation—Methods applied for wastewater treatment. Environ. Eng. Manag. J. 2005, 4, 463–472.
- Daneshvar, N.; Sorkhabi, H.A.; Tizpar, A. Decolorization of Orange II by electrocoagulation method. Separ. Purific. Technol. 2003, 31, 153–162.
- Ramesh Babu, B.; Parande, A.K.; Raghu, S.; Prem Kumar, T. Textile technology. Cotton Textile processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153125.
- Wei, Y.; Ding, A.; Chen, Y. Removal of refractory dyes by a novel chlorine-free coagulant of polyferric-silicate-acetate (PFSA): Characterization and performance evaluation. J. Environ. Chem. Eng. 2022, 10, 108524.
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin. Water Treat. 2015, 108, 9203–9215.
- Huang, X.; Wan, Y.; Shi, B.; Shi, J.; Chen, H.; Liang, H. Characterization and application of poly-ferric-titanium-silicate-sulfate in disperse and reactive dye wastewaters treatment. Chemosphere 2020, 249, 126129.
- Wei, Y.; Cheng, X.; Ding, A.; Xu, J. Magnesium silicate polymer as a coagulant for reactive dye removal from wastewater: Considering the intrinsic pH in magnesium silicate polymer and coagulation behavior. ACS Omega 2020, 5, 26094–26100.
- Pang, F.M.; Kumar, P.; Teng, T.T.; Mohd Omar, A.K.; Wasewar, K.L. Removal of lead, zinc and iron by coagulation-flocculation. J. Taiwan Inst. Chem. Eng. 2011, 42, 809–815.
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Y. Effect of viscosity, basicity and organic content of composite flocculant on the decolorization performance and mechanism for reactive dyeing wastewater. J. Environ. Sci. 2011, 23, 1626–1633.
- Yeap, K.L.; Teng, T.T.; Poh, B.T.; Morad, N.; Lee, K.E. Preparation and characterization of coagulation/flocculation behavior of a novel inorganic–organic hybrid polymer for reactive and disperse dyes removal. Chem. Eng. J. 2014, 243, 305–314, ISSN 1385-8947.
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, T. An application study of inorganic-organic composite polymer in flocculating reactive dye wastewater under different conditions. Int. Conf. Biol. Environ. Chem. 2011, 24, 139–143.
- Su, C.X.; Teng, T.T.; Morad, N.; Rafatullah, M. Optimisation of the coagulation-flocculation of reactive dye wastewater using novel inorganic-organic hybrid polymer. Iran. J. Energy Environ. 2016, 7, 31–38.
- Lee, K.E.; Teng, T.T.; Morad, N.; Poh, B.T.; Mahalingam, M. Flocculation activity of novel ferric chloride-polyacrylamide (FeCl3-PAM) hybrid polymer. Desalination 2011, 266, 108–113.
- Lee, K.E.; Goh, T.L.; Simon, N. Textile industrial wastewater treatment by polyacrylamide aided magnesium chloride hybrid coagulant. Nat. Environ. Pollut. Technol. 2017, 16, 399–407.
- Al-Ani, Y.; Li, Y. Degradation of C.I. Reactive Blue 19 using combined iron scrap process and coagulation/flocculation by a novel Al(OH)3-polyacrylamide hybrid polymer. J. Taiwan Inst. Chem. Eng. 2012, 43, 942–947.
- Lee, K.E.; Hanafiah, M.M.; Halim, A.A.; Mahmud, M.H. Primary treatment of dye wastewater using aloe vera-aided aluminium and magnesium hybrid coagulants. Procedia Environ. Sci. 2015, 30, 56–61.
- Wu, J.; Xue, Y.L.; Yang, G.; Sun, B.Y.; Zhang, L. Preparation and performance of polysilicate aluminum ferric-chitosan. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4.
- Abd-Elhakeem, M.A.; Alkhulaqi, T.A. Simple, rapid, and efficient water purification by chitosan coated magnetite nanoparticles. J. Environ. Nanotechnol. 2014, 3, 17–20.
- Sun, Y.; Zhou, S.; Sun, W.; Zhu, S.; Zheng, H. Flocculation activity and Evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Separ. Purific. Technol. 2020, 1016, 116737.
- Zhou, L.; Zhou, H.; Yang, X. Preparation and performance of a novel starch-based inorganic/organic composite coagulant for textile wastewater treatment. Separ. Purific. Technol. 2019, 210, 93–99.
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665.
- Wang, S.; Kong, F.; Fatehi, P.; Hou, Q. Cationic High Molecular Weight Lignin Polymer: A Flocculant for the Removal of Anionic Azo-Dyes from Simulated Wastewater. Molecules 2018, 23, 2005.
- Cao, B.; Yue, Q.; Miao, J. Evaluation of polyaluminium ferric chloride (PAPC) as a composite coagulant for water and wastewater treatment. Water Sci. Technol. 2003, 47, 127–132.
- Gao, B.Y.; Wang, Y.; Yue, Q.Y. The chemical species distribution of aluminium in composite flocculants prepared from polyaluminium chloride (PAC) and polydimethyldiallylammonium chloride (PDMDAAC). Acta Hydrochim. Hydrobiol. 2005, 33, 365–371.
- Liu, Z.M.; Sang, Y.M.; Tong, Z.G.; Wang, Q.H.; Sun, T.C. Decolourization performance and mechanism of leachate secondary effluent using polyaluminium (III)-magnesium (II) sulphate. Water Environ. J. 2012, 26, 85–93.
- Zhao, H.; Peng, J.; Xue, A.; Ni, J. Distribution and transformation of Al species in organic silicate aluminium hybrid coagulants. Compos. Sci. Technol. 2009, 69, 1629–1634.
- Dong, Y.; Deng, A.; Guo, H.; Tang, X. Preparation and flocculation performance of a polyacrylamide/montmorillonite hybrid flocculant. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2009, 29, 2385–2392.
- Nanko, M. Definitions and categories of hybrid materials. Azojomo 2009, 6, 1–8.
- Zaharia, C. Coagulation-flocculation processes in water and wastewater treatment. (II) Fine particles and its removal using electrolytes and polyelectrolytes. In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; ‘Alexandru Ioan Cuza’ University Publishing House: Iasi, Romania, 2014; Volume III, pp. 165–194.
- Moussas, P.A.; Zouboulis, A.I. A new inorganic-organic composite coagulant, consisting of polyferric sulphate (PFS) and polyacrylamide (PAM). Water Res. 2009, 43, 3511–3524.
- Wang, Y.; Hao, B.; Yue, Q.; Wei, J.; Li, Q. The characterization and flocculation efficiency of composite flocculant iron salts-polydimethyldiallylammonium chloride. Chem. Eng. J. 2008, 142, 175–181.
- Sen, G.; Kumar, R.; Ghosh, S.; Pal, S. A novel polymeric flocculant based on polyacrylamide grafted carboxymethyl starch. Carbohyd. Polym. 2009, 77, 822–831.
- Lee, K.E.; Khan, I.; Morad, N.; Teng, T.T.; Poh, B.T. Thermal behaviour and morphological properties of novel magnesium salt-polyacrylamide composite polymers. Polym. Compos. 2011, 32, 1515–1522.
- Sun, T.; Sun, C.H.; Zhu, G.I.; Miao, X.J.; Wu, C.C.; Lv, S.B.; Li, W.J. Preparation and coagulation performance of poly-ferric-aluminium-silicate-sulphate from fly ash. Desalination 2011, 268, 270–275.
- Gao, B.Y.; Yang, Y.; Yue, Q.Y.; Wei, J.C.; Li, Q. Color removal from simulated dye water and actual wastewater using a composite coagulant prepared by polyferric chloride and polydimethyldiallylammonium chloride. Sep. Purif. Technol. 2007, 54, 157–163.
- Zhao, y.; Zhang, L.Y.; Ni, F.; Xi, B.; Xia, X.; Peng, X.; Luan, Z. Evaluation of a novel composite inorganic coagulant prepared by red mud for phosphate removal. Desalination 2011, 273, 414–420.
- Huang, P.; Xia, D.; Kazlauciunas, A.; Thornton, P.; Lin, L.; Menzel, R. Dye -mediated interactions in chitosan-based polyelectrolyte organoclay hybrids for enhanced adsorption of industrial dyes. ACS Appl. Mater. Interfaces 2019, 11, 11961–11966.
- Jagaba, A.; Birniwa, A.H.; Usman, A.; Mu’azu, N.; Yaro, N.; Usman, A.K.; Mu’azu, N.D.; Yaro, N.S.A.; Soja, U.B.; Abioye, K.J.; et al. Trend and current practices of coagulation-based hybrid systems for pulp and paper mill effluent treatment: Mechanisms, optimization techniques and performance evaluation. J. Clean. Prod. 2023, 429, 139543.




