1. Textile WW Treatment Processes for Dye(s) and Color Removal
Equalization and sedimentation were considered viable as preliminary treatment operations in the primary mechanical treatment step of textile WWs until 1990; they were used for dye and color removal since no limits were imposed on their content in the treated effluent
[1][2][3,54]. The primary treatment step was usually followed by the secondary step based on specific biological–mechanical processes. After the approval of restrictive/limitative quality standards for treated effluent discharged to different aquatic receptors, technological treatment processes were introduced that were available and efficient techniques used for color and dye removal. The majority were based on discoloration and degradation processes
[3][4][5][6][7][8][9][10,55,56,57,58,59,60], which are active in the
primary (physical–chemical–mechanical treatments based on precipitation, coagulation, flocculation, sedimentation, filtration, or air flotation, etc.),
secondary (biological–mechanical treatments based on biodegradation, adsorption, redox and/or ionic exchange, sedimentation, and filtration), and
tertiary (advanced physical–chemical treatments based on advanced oxidation, precipitation, membrane processes, filtration on multiple adsorptive materials’ layers, etc.) treatment steps but also in
sludge treatment (achieved via supervised tipping, chemical–mechanical conditioning, recycling, especially for sludge dehydration, or even incineration).
Discoloration processes are especially used in removing colors from textile WWs, but the treated effluent can remain loaded with a significant content of organics expressed through high values of COD-Cr, BOD5, and/or TOC, commonly exceeding the standard limits. For both color and dye removal from textile effluents, degradation processes are prominently used, which are processes involving the destruction of complex dye and auxiliary organic structures based on decomposition or chemically breaking down dye molecules into small molecular structures, i.e., degradation products such as carbon dioxide, water, simple minerals, and organic by-products (e.g., organic acids and alcohols, small organics with low molecular weights).
All states of the European Community enforce strict legislative norms and measures concerning the presence of coloring and dyes in WW produced within the community and natural water resources; however, there is no official document that lists the different limits of numerous classes of dyes present in treated effluent, only a limitative sum of dye (<1 mg/L) and color (<10–50 HU) content that can be present in the treated effluent, which is dependent on a country’s strategic policy and the norms imposed by environmental authorities/regulators on the compliance plan of certain textile companies. Moreover, in UK, a law that declares ‘no synthetic chemicals should be discharged into the marine environment’
[2][54], including synthetic dyes, is still active.
In recent years, the E.U., Canada, USA and Australia have permitted environmental legislative authorities to specify the threshold concentration levels of different polluting species in treated WW, including dyes. Morocco and Turkey use the EU model, while Thailand adopted the USA system. In Pakistan, India, and Malaysia, effluent discharge limits are regulated by specific directives of their Central Pollution Control Board, but the limits for azo dyes are not specified, and other dye classes are considered separate groups, unrelated to the other physical-chemical characteristics of treated WW (e.g., the total dissolved solids (TDSs) content or color index)
[7][8][9][10][11][12][13][14][15][16][58,59,60,61,62,63,64,65,66,67].
The scientific literature reports various treatment schemes for textile WWs while referring to their initial compositions, but an important indicator when forming a decision is the COD
Cr/BOD
5 ratio, which estimates the biodegradability of WW
[7][8][9][10][11][12][13][14][15][16][17][18][19][20][21][58,59,60,61,62,63,64,65,66,67,68,69,70,71,72]. If this ratio is <2.5, the colored effluent contains at least 40% biodegradable organic compounds that are susceptible to biological treatments (which are efficient, commonly used, and low-cost)
[22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91]. If the ratio is between 2.5 and 3.5, a comparative study of biological and physical–chemical treatment process efficiency is required (various treatment processes can be applied); if the ratio is >3.5, physical–chemical processes are mostly indicated (at least as a pre-treatment before the application of a biological treatment step) due to the high fraction of non-biodegradable compounds
[7][8][9][41][42][43][44][58,59,60,92,93,94,95]. Commonly, technological treatment processes of colored effluents can use different methods that are grouped as follows: (i)
conventional methods: coagulation/flocculation, precipitation, biodegradation, sand filtration (lent or rapid), and adsorption using activated carbon (AC); (ii)
recovery methods: solvent extraction, evaporation, oxidation, electrochemical treatment, membrane separation, treatment in the membrane bioreactors, ion exchange, and incineration; and (iii)
emerging removal methods: advanced oxidation, adsorption onto non-conventional solids (e.g., ‘low cost’ adsorbents), biosorption, living biomass growth, nanofiltration, etc.
[2][4][5][6][7][8][9][10][11][12][13][14][15][16][17][18][19][34][35][36][37][38][39][40][45][12,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,85,86,87,88,89,90,91].
A better and deeper understanding is needed that considers all biological, physical, ecological, social, and economic interactions or other connections surrounding a textile WW treatment system. The techniques used to describe and solve the concerns and treatment problems are those used by chemical, biochemical, hydrological, environmental and/or technological engineers who select the adequate technological process in terms of cost-efficiency, develop models based on mass balances or conversion efficiencies relating to different technological treatment steps, minimize residual concentrations of different polluting ionic and molecular species in the treated WWs or water resources considered for supply or discharge, and avoid the development of health effects or the dispersion of toxic compounds.
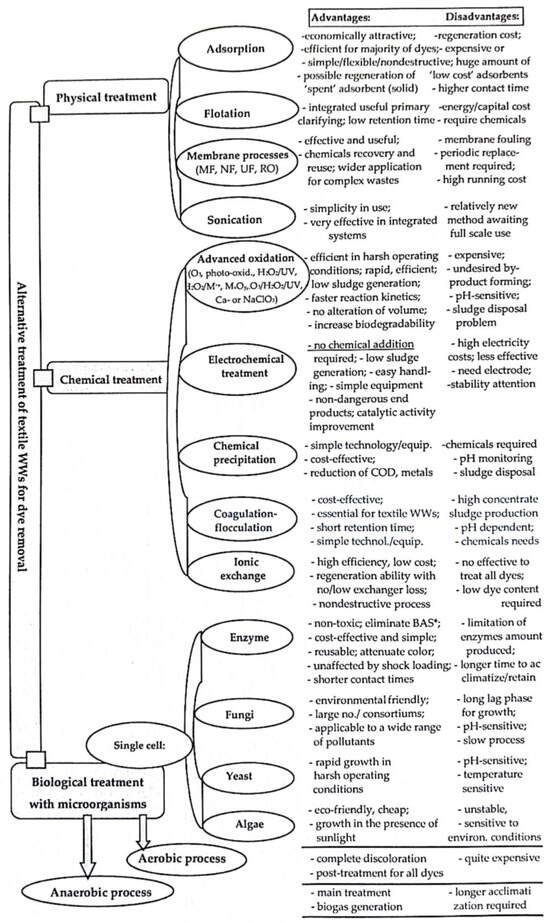
Some treatment processes used for colored WW that are commonly applied to textile effluents are summarized in
Figure 12 in association with their principal advantages and disadvantages. Each treatment process has specific constraints considering the treatment cost, feasibility, efficiency, practicability, reliability, environmental impact, sludge production, difficulties in operation and processing, pre-treatment requirements, and the possibility of producing potentially toxic by-products
[45][46][47][48][12,41,42,43].
Figure 12. Alternative treatment processes applied to colored textile WW. (* BAS—biochemical oxygen demand and suspended solids).
In general, physical–chemical and biological treatments can remove most pollutants from industrial effluents, but it is important to concentrate research on cheaper and more effective combined/mixed treatments or new alternatives that can be possibly applied in each country (impoverished, developing, or even highly industrialized nations) of our changing world. Therefore, biological treatments are based on biodegradable conversion processes of contaminants/pollutants from colored WW into more simple and harmless products with the help of different groups of microorganisms (bacteria, fungi, yeasts, and algae) (via adsorption and biodegradation with the help of active bacteriological biomass). These methods produce less sludge and also require fewer chemicals, are economically feasible in poor and developing countries (no large investment and operational costs), have energy-saving features, and permit the complete mineralization of the dyes
[1][3][6][18][19][20][45][3,10,12,57,69,70,71].
The chemical processes used for dye removal from dye-containing effluents are often more expensive than physical and biological treatments (except electrochemical techniques) and require chemicals, specific equipment, and electricity and can produce toxic by-products (secondary metabolites) that entail additional disposal and treatment problems
[1][3][21][22][45][3,10,12,72,73].
The representative chemical treatments are as follows:
(i) advanced oxidation processes (AOPs) operating in the presence of UV light and/or oxidizing agents (hydroxyl radicals, persulphate radicals, ozone, hydrogen peroxide, and other oxidizing agents in association or not with various catalysts) under specific conditions of temperature and pressure as stand-alone or hybrid technologies can be used
[23][24][25][26][27][28][74,75,76,77,78,79];
(ii) coagulation processes operating under vigorous mixing conditions for the charge neutralization of fine particles in WW followed by flocculation under gentle mixing for fine solid agglomeration, and further floc separation via sedimentation can be applied. They are natural and synthetic coagulants/flocculants of inorganic and organic natures; recently, however, increasing interest has been on the development of hybrid materials (of inorganic–inorganic, inorganic–organic, organic–natural, inorganic–natural, or inorganic–organic–natural origin)
[9][29][30][31][42][43][60,80,81,82,93,94];
(iii) electrochemical treatments can be used that operate in electrochemical cells/reactors with two metal electrodes connected at a direct current source in which the coagulant is in situ generated at the anode and hydrogen gas evolves at the cathode
[32][33][34][83,84,85]. In addition, electro-Fenton and anode oxidation are considered electrochemical treatments that enable the removal of dyes in two stages based on a combination of oxidation and coagulation processes named usually electrochemical AOPs
[35][36][37][86,87,88];
(iv) ion exchange treatments developed based on the strong interactions between the functional groups of ion exchange resins and charged dyes are highly efficient, low-cost methods but can only be used for low and medium concentrations of contaminants
[3][45][10,12].
The physical processes used for the removal of dye from colored effluents involve mass transfer processes and are low-cost treatments with high efficiency (85–99%), simple designs, easy operating units, fewer chemical requirements, and no inhibitory effect due to the presence of toxic species. Such physical processes are adsorption, membrane filtration as microfiltration (MF), ultrafiltration (UF), nanofiltration (NF), reverse osmosis (RO), which are all known to be membrane separation processes that remove contaminants from WW; the difference between these separation processes especially relating to pores size, which can be 0.1–10 μm (MF), 0.001–0.1 μm (UF), and 0.5–2.0 nm (NF)
[38][39][40][89,90,91].
In the case of certain textile effluents, the scientific literature reports that WW treatments can be commonly based on the following three steps:
[3][10][11][12][10,61,62,63]: (i) a coarse prefiltration of mixed textile effluents to remove any fibrous matter; (ii) the chemical treatment of the effluent with a 10% lime slurry into a first tank to yield a pH of 11.30, after which an iron (II) sulphate solution can be added into a second tank, forming a precipitate of iron (II) hydroxide, and finally, the addition of polyelectrolyte to aggregate the floc particles into a third tank; and (iii) S/L separation via a faster and more effective particles settlement in the settlement tank; the sludge is automatically pumped to a sludge tank using a vacuum extraction device, and the supernatant passes through two further tanks where the pH is adjusted to values between 7.5 and 8.5 by injecting carbon dioxide under pressure or, in some instances, concentrated hydrochloric acid. The treated effluent is further pumped to the biological plant for treatment
[10][11][12][61,62,63].
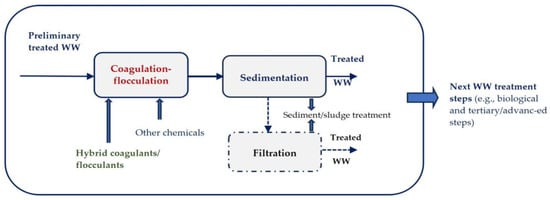
The abovementioned technological treatment process is successful in decolorizing strongly colored dyehouse effluent. Frequently, the agglomeration of fine solids to settle or improve filtration (e.g., single or multi-bed filtration) requires only the action of an electric or magnetic field due to the indirect formation of specialized coagulation–flocculation agents (via dissolution on different iron- or aluminum-based electrodes or the specific action of metal species present in WWs). Commonly, there are necessary specific chemical materials that need to be introduced to the colored WW to achieve the coagulation and flocculation processes for high and even the complete removal of small suspended and colloidal particles of various compositions. Chemical precipitation and coagulation–flocculation processes remove more than 50% of the BOD5 of the raw effluent but the decolorized effluent has COD-Cr and BOD5 loads that are too high for discharge into the watercourse (river, lake, or lagoon); therefore, additional treatment steps are required to fulfil the compliance plan requirements for discharge limits of all of the imposed WW quality indicators. Usually, typical mechanical–chemical–biological technological treatment processes for textile effluents consist of the following steps: WW collection and storage (preliminary step), equalization (WW mixing and cooling step), pH adjustment, coagulation–flocculation + aggregate/floc separation, biological treatment (bio-oxidation) + sludge separation (sludge thickening), filtration, and the disinfection and discharge of the treated WW. The chemical treatment step based on coagulation–flocculation with aggregates/flocs separation reduces the turbidity, suspended solids, oil, organic matter, color, and COD/BOD ratio, as illustrated in Figure 23.
Figure 23. Coagulation–flocculation treatment step for colored colloids separation from WWs.
2. Coagulation–Flocculation Technologies and Its Performances in Colored Textile WWs Treatment
The scientific literature clearly indicates that colored colloids from textile effluents cannot be separated via simple gravitational means, and some chemicals (e.g., lime, ferrous and ferric salts, aluminum salts, various polymers containing these metallic ions, such as poly aluminum chloride (PAC), poly aluminum sulphate (PAS), or cationic, anionic, and nonionic organic polymers/co-polymers (polyelectrolytes) based on poly acrylamide, polyacrylic acid, polyimides, other macromolecular structures, such as processed hybrid materials of inorganic, organic, and mixed/hybrid origins)
[9][10][11][12][13][14][15][16][17][18][19][20][41][42][43][44][49][60,61,62,63,64,65,66,67,68,69,70,71,92,93,94,95,96] are added to cause the fine solids to separate via settlement or/and filtration. These chemicals destabilize colloidal suspended small particles (e.g., dyes, clay, iron, heavy metals, organic solids, and oil in WW) and emulsions entrapping solids (coagulation) and/or lead to the agglomeration of these particles to flocs large enough to settle (flocculation) or highly improve further filtration/biofiltration
[9][13][14][15][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37][38][39][40][41][45][49][50][51][52][53][54][55][56][57][12,60,64,65,66,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,96,97,98,99,100,101,102,103,104]. Anionic, nonionic, and cationic polymers can be used for the flocculation process. If an over dosage with coagulants and/or flocculants was applied, it is possible to intervene in a particle charge reversal due to the adsorption of excess ions, and thus stable colloid particles are presented
[9][41][49][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][60,92,96,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123].
The scientific literature has reported
four major coagulation–flocculation mechanisms to explain the agglomeration of colloidal particles at higher particle size dimensions and, consequently, the ease of separation via gravity sedimentation or filtration
[9][41][42][43][44][49][50][51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][60,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150]:
(
i)
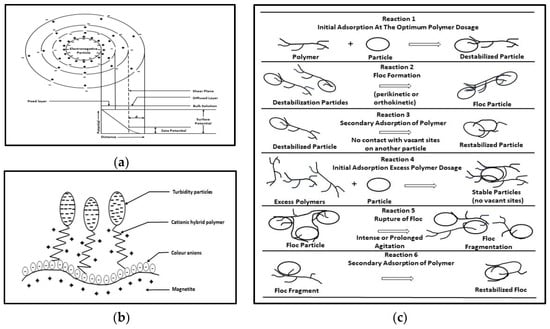
double layer compression: The colloids present in WW are commonly charged and often described in terms of an electrical double layer. The charge serves to attract opposite counterions from the surrounding aqueous medium and, thus, it forms a layer adjacent to the colloid surface, which leads to ion exchange properties (
Figure 34a). The stability of colloids and suspended solids in water is maintained because the small, charged particles repel each other. If they come sufficiently close, the repulsive forces of the surface charge are balanced by the attractive forces of counterions, and aggregation can occur with the formation of larger and settable flocs. One destabilization mode occurs when there is a high concentration of electrolyte in the water (acting as a coagulant), representing a source of counterions that accumulate around the solid surface and reduce the thickness of its double layer. The space charge density of ions in water rapidly decreases with distance from the particle surface, and the potential exponentially declines as a function of distance (DLVO model: Derjaguin–Lindau–Verney–Overbeek). For the concentration of monovalent electrolyte (NaCl) in water of 0.1 M and 10
−3 M, the double layer thickness is of 11 Å and 101 Å, and, in the case of certain salts, small concentrations may be enough for particle aggregation. The double layer thickness decreases markedly with increasing counterion valence (Hardy rule). Therefore, the interaction energy (
ET) is defined as follows:
where
EA is the van der Waals attraction energy, and
ER is the coulombic repulsive energy of the double layer.
Figure 34. Schematic representation of coagulation–flocculation mechanism. (
a) Electric double layer of a colloidal particle; (
b) interaction mechanism between colloids, colored species, and polymer-based coagulants; (
c) flocculation mechanism with polymer-based hybrid materials
[44][49][103][95,96,150].
The critical concentration of the coagulant (commonly a metal-containing salt or a certain metal-containing hybrid material) used for the aggregation of colloidal particles depends on the co-ions rather than the counter ions. The Hofmeister series rule must be considered that relates to coagulation effectiveness as SO42− > Cl− > NO3− > I−.
(ii) charge neutralization and adsorption: The retention of species on colloid surfaces can be produced via charge neutralization and specific binding. Adsorption phenomena (physical process) is based on electrostatics (coulombic electrostatic forces that are weak in comparison to covalent, coordinative, or hydrogen bonds) when the charge density on both the colloid and water/WW species determines the extent of adsorption. The colloid–coagulant, coagulant–WW, and colloid–WW interactions are important when compared with coulombic energy (i.e., colloids–surfactant-like molecules such as dodecyl ammonium chloride). When enough counterions are adsorbed, charge reversal takes place, and re-stabilization occurs. If long-chain counterions (polymers, such as polyelectrolytes, and certain polymeric hybrid materials) are attached to the colloid surface, the effective charge outside of the shear layer is reduced in contrast to double-layer repression (double-layer compression), which alters the charge distributions within the diffuse layer (Figure 34b).
(iii) entrapment in a precipitate (co-precipitation) and adsorption: The addition of electrolytes as coagulants (e.g., Fe3+ and Al3+ salts) in colloid-containing WW leads to the formation of polynuclear hydrolysis products, such as M(OH)nz+, which are adsorbed at solid–WW interfaces (e.g., hydrous metal oxide interfaces). It is possible that the formation of a surface complex is due to cation and anion adsorption at hydrous colloid interfaces and the establishment of ligand exchange equilibrium, e.g., the coordination/complexation of cations and anions onto amphoteric hydrous metal oxides.
(iv) interparticle bridging: Polyelectrolytes (e.g., polymer/co-polymer/or macromolecular compounds with multiple ionizable functional groups on its chain, which are soluble in water and have macromolecular structures with flexible chains and charges) and certain polymeric hybrid materials can modify the surface of mineral solids, leading to floc formation. The use of excess polymer, prolonged agitation, or a lack of intramolecular adsorption onto solids can re-stabilize colloidal systems (Figure 34c).
In practice, all four mechanisms can act on a system, but the first two mechanisms are implicated in solid particle neutralization and agglomeration, and the last two are responsible for the growth in aggregate size and/or aggregation as a flocculation process (
Figure 34). In the case of using hybrid materials as coagulants/flocculants, all mechanisms act together and depend on the type, composition (metal-containing species and charged functional groups of organic polymeric chains and metal salt content), WW characteristics, and imposed operating conditions. As a result, the coagulation–flocculation process applied in colored WW treatment can act according to the following
[20][58][70][71][72][73][74][75][76][71,105,117,118,119,120,121,122,123]:
- –
-
Attraction forces: these decrease the surface charge and enhance the aggregation of solids in distinct, separable aggregates or flocs (coagulation process).
-
- –
-
The simple electrostatic adsorption of counterions: effectively neutralizes the solid surface charge and decreases the surface potential (dependent on ionic species or large, complex molecules, and ordinary adsorption) (coagulation–flocculation process).
-
- –
-
Precipitation: hydrated metal hydroxides (precipitates) are formed that can adsorb on the solid surface with other existing colloids and neutralize the surface charge (pH-sensitive, with the characteristic value of the isoelectric point of metal hydroxide) (coagulation–flocculation process).
-
- –
-
Enmeshment in an agglomerate precipitate and adsorption: is when organic polymers are used (cationic, anionic, and nonionic ones). The existing ions in the WW interact with the polymeric chains, forming solid aggregates (flocculation process).
-
Flocculation usually refers to the post-destabilization process in which large flocs are produced as a result of the collision of small aggregates due to rapid stirring (peri-kinetic flocculation via Brownian motion) or slow stirring (orthokinetic flocculation via velocity gradients). Both rapid and slow stirring are required for good and complete flocculation with conventional coagulants/flocculants
[44][49][50][95,96,97]. When hybrid materials are applied as coagulant–flocculants, a single stirring regime can be used at an adequate constant velocity, yielding increased treatment efficiency.
Commonly, the separation of dense produced aggregates or flocs from WW is achieved by (i) sedimentation, which is the settling of the flocs without stirring for quiescence settling or (ii) filtration/biofiltration, which is the separation of flocs using a free vacuum or under pressure by passing them through a granular solid layer of certain porosity and density (e.g., graded sand, garnet, coal, and resins) supported by gravel layers and/or porous underdrains (depth filters) or precoat filters
[76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149].
The efficiency of WW coagulation–flocculation processes in relation to solid separation via sedimentation is important. The highest removal of suspended solids and turbidity after this WW treatment step must be higher than 60–90%, ideally 100% (in a mechanical–chemical WW treatment system). In practice, the effect of coagulation–flocculation on sedimentation performance is beneficial, improving the removal of suspended solids and turbidity but not completely (48–92%)
[9][20][45][49][58][76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][12,60,71,96,105,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159].
The main disadvantage of this WW treatment step is that the process control is a little difficult, possibly due to precipitation rate and floc size growth involving contaminants or residuals such as non-ionic detergents remaining in the effluent, sludge production, which must be settled, dewatered, and pressed into a cake for subsequent landfill tipping, the necessity for further detoxifying and valorization (useful compounds recovery), or incineration
[51][52][53][54][55][56][57][58][59][60][61][62][63][64][65][66][67][68][69][70][71][72][73][74][75][76][98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123].
Very effective chemical coagulation–flocculation (C-F) methods and the precipitation of phosphorus and existing carbonates in different colored WWs (e.g., textile WWs) have been reported, which reduce the load of the biological treatment, working with relatively high concentrations of inorganic coagulants based on lime, iron, and aluminum salts (e.g., more than 200–300 mg/L); however, very good results were also reported when using a combination of an inorganic coagulant and a polymeric flocculant (coagulation aid), such as for a reference model of textile WWs reported in the scientific literature, such as a poly (aluminum chloride) (PAC) along with an organic polymer
[70][117] or ferrous/ferric chloride and a commercial organic coagulant aid (e.g., sodium alginates) at a pH of 6.7–8.3 (color removal > 80%)
[71][118], alum at pH = 8.2 (54–81% color removal) with the addition of bentonite (3 g/L) for Remazol Violet dye-containing effluent
[72][119], or ferric chloride and two commercial polyelectrolytes, cationic Prodefloc CRC 301 and anionic Ponilit GT-2 polyelectrolyte at a pH of 7.38–7.83 (turbidity removal of >86.12%, color removal of 48.22%, and COD removal of >36.84%)
[12][63].
In the case of industrial beverage WW treatment applied for the removal of trace metals, such as total Fe (Fe
2+ + Fe
3+), total Cr (Cr
3+ + CrO
42−) and Zn (Zn
2+) ions, the effectiveness of polymer/co-polymer or hybrid material addition to the coagulation–flocculation process was verified, especially when both FeCl
3 (300 mg/L) and an organic polymer (a non-ionic polyacrylamide, 65 mg/L) were added individually, using a FeCl
3–polymer/co-polymer combination (hybrid material); in the case of the individual use of a ferric-based coagulant, high removals of metal species were reported, such as total Cr(III, VI) ion removal (91%), Zn(II) (72%), and total Fe(II, III) (54%), and the addition of polymer/co-polymer increased the efficiencies of the processes to about 95%, 87%, and 88%, respectively. Another case is that of synthetic WWs (models of industrial WW from cosmetic manufacturing) containing Cu
2+, Ni
2+, Zn
2+, and Pb
2+ ions together with vegetal oil (cedar oil), which were treated with inorganic precipitation agents (sodium carbonate and lime) at pH = 8.5–9.3 and anionic Ponilit GT-2 polyelectrolyte (0.25–0.75 mg/L) individually or in association with anionic Ponilit GT-4 polyelectrolyte as flocculants (co-polymers based on maleic acid, acrylic aldehyde and/or acid)
[73][120] by using chemical precipitation and coagulation–flocculation followed by air flotation and rapid filtration with relatively to very good removals of 54.60–96.20% for Cu(II), 51.52–96.10% for Ni(II), 68.68–96.80% for Zn(II), 68.90–96.08% for Pb(II), and 82.30–98.30% for oil
[74][121].
Numerous scientific reports have noted that the combination of ferric chloride and polymer/polymeric hybrid materials at different ratios achieved high removal efficiency in relation to the removal of metal species and color from WW
[76][77][78][79][80][81][82][83][84][85][86][87][88][89][90][91][92][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109][110][111][112][123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146,147,148,149,150,151,152,153,154,155,156,157,158,159].
In the presence of an electric field, the efficiency of the coagulation–flocculation processes improves due to the good separation of the S/L phases using electrochemical processes, especially electrocoagulation–electro flotation, in which multiple processes are involved, such as electrolytic reactions at the electrodes, the formation of coagulants when treating the effluent, the adsorption of soluble or colloidal pollutants on solid coagulants, and removal after settlement or dissolved air flotation
[12][13][14][75][63,64,65,122]. This electrochemical treatment is efficient even at high pH for color and COD-Cr removals, and it is strongly influenced by the current density and duration of the reaction. The EC treatment was applied with high efficiency for textile WWs. Thus, the EC efficiency in WW containing Orange II and Acid Red 14 dye was found to be higher than 98% for color removal
[76][77][123,124], and in industrial effluent containing Yellow 86, high turbidity, COD-Cr, and extractible substances were 87.20%, 49.89%, 94.67%, and 74.20% after 30 min of operation at a current intensity of 1 A with monopolar electrodes
[75][122], where iron was the sacrificial anode (producing an iron-based coagulant). Discoloration performance in EC treatment was reported to be in the range of 90–95%, and for COD-Cr, it was reported to be in the range of 30–46% under optimal conditions
[49][96].
The same efficient effect has been noted for magnetic fields in relation to the separation of different solid agglomerates (agglomerated metal co–precipitates) from WWs, especially new metal-based formed aggregates that are easily separable from treated WW with the help of efficient magnets (Figure 34b). In both electric and magnetic fields, the WW treatment process can be performed using advanced electrochemical or magnetic separation processes with very good results, usually considered recovery treatment methods.