Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Tsvetelina Velikova | -- | 2041 | 2024-03-07 10:27:10 | | | |
| 2 | Sirius Huang | Meta information modification | 2041 | 2024-03-08 02:36:43 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mileva, N.; Velikova, T.; Velikov, T.; Vassilev, D. Aortic Elasticity and Cardiovascular Risk Stratification. Encyclopedia. Available online: https://encyclopedia.pub/entry/55964 (accessed on 07 February 2026).
Mileva N, Velikova T, Velikov T, Vassilev D. Aortic Elasticity and Cardiovascular Risk Stratification. Encyclopedia. Available at: https://encyclopedia.pub/entry/55964. Accessed February 07, 2026.
Mileva, Niya, Tsvetelina Velikova, Toni Velikov, Dobrin Vassilev. "Aortic Elasticity and Cardiovascular Risk Stratification" Encyclopedia, https://encyclopedia.pub/entry/55964 (accessed February 07, 2026).
Mileva, N., Velikova, T., Velikov, T., & Vassilev, D. (2024, March 07). Aortic Elasticity and Cardiovascular Risk Stratification. In Encyclopedia. https://encyclopedia.pub/entry/55964
Mileva, Niya, et al. "Aortic Elasticity and Cardiovascular Risk Stratification." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Cardiovascular risk stratification is a cornerstone of preventive cardiology, aiming to identify individuals at a higher risk for adverse events. In line with this, aortic elastic properties have gained recognition as crucial indicators of vascular health and predictors of cardiovascular outcomes.
aortic elasticity
stiffness
compliance
cardiovascular risk
vascular health
atherosclerosis
1. Introduction
The assessment of aortic elastic properties stands at the intersection of cardiovascular research, diagnosis, and risk stratification. The aorta, a vital conduit of the circulatory system, is heavily burdened in maintaining pulsatile blood flow, buffering cardiac pulsations, and reducing afterload on the left ventricle. During each cardiac cycle, the left ventricle (LV) ejects blood into the aorta, generating a high-pressure wave. However, the pulse wave is not directly transmitted to the smaller vasculature but is initially transformed in the aorta. The elastic properties of the large arteries enable the expansion of the vessel in order to accommodate the stroke volume and attenuate e the high-pressure pulsations. This aortic distention protects the smaller arteries and capillaries from the pulsatile pressure that may injure their fragile structure.
Therefore, disturbances in aortic elastic properties, characterized by stiffness, compliance, and distensibility changes, have been considered as important factors in the pathogenesis of numerous cardiovascular pathologies [1][2]. These comprise various disorders such as arterial hypertension, atherosclerosis, acute and chronic aortic syndromes, and valvular heart diseases [1]. If the aortic elasticity is compromised, it loses its ability to diminish the high pulsatile pressure developed in the left ventricle, therefore exposing the cardiovascular system to heightened stress.
2. Physiology of Large Arteries
One of the main characteristics of the elastic arteries is that their medial layer is rich in collagen and elastin filaments. This quality enables the stretch in response to ventricular contractions [3]. It has been revealed that the thoracic aorta contains up to 40% elastic filaments. However, the amount of elastin decreases with the reduction of the vessel size in peripheral circulation [4]. Reduced elasticity has been proved in the segments of the aorta from the proximal to the distal vessel parts [5]. The elastic arteries during systole store almost half of the LV stroke volume. In contrast, in diastole, the aortic wall elastic forces release this residual volume to the periphery vessels continuously, ensuring a stable peripheral circulation. This hemodynamic process has been described as the Windkessel effect [6]. During LV systole, the heart ejects about 60–100 mL of blood into the aorta and arteries, with relatively 50% of the stroke volume being forwarded directly to peripheral circulation. However, the aorta acts as an elastic reservoir, storing the other 50% of the stroke volume [4]. With the fall in aortic pressure in diastole, the aorta recoils gradually, and the stored blood volume is ejected into peripheral circulation. Therefore, pressure and blood flow are maintained throughout diastole, and a relatively continuous peripheral flow is provided despite the pulsatile myocardial contractions. Thus, the elastic properties of the large arteries enable the sustained pressure in the arteries despite the alternating left ventricular ejection and the pulsatile blood flow. Apart from peripheral circulation, this physiologic process also affects the heart, by reducing the LV afterload, which leads to an improvement in coronary blood flow and left ventricular relaxation [7]. One other mechanism necessary for maintaining adequate diastolic pressure is that, usually, in a healthy organism, the pulse wave velocity in the large arteries is rather slow. The reflection of this signal in peripheral circulation, and the return of the wave to the ascending aorta forms the dicrotic wave during early diastole [8].
The Windkessel effect depends on the elastic properties of the aorta [6]. The physics definition of elastic materials are substances that readopt their initial shape after the application of an external force. During a myocardial contraction, the ejected stroke volume kinetic energy is first converted into potential energy within the ballooned aortic wall [6]. Afterwards, the stored potential energy is transformed into kinetic energy during diastole when the aorta gradually recoils [7]. Therefore, despite the diastolic cessation of the heart’s contraction, the amount of blood within the peripheral arteries does not reach a diastolic halt, and blood pressure does not decrease to null.
3. Concept of Elasticity, Stiffness, and Compliance
The aortic elastic properties include both the ability to distend due to the increased pressure and the ability to recoil gradually to the initial vessel size as the blood pressure reduces in diastole. The vessels’ elastic resistance (elastance) explains the resistance in which the large arteries push against their distention in a situation where an additional volume is ejected and when the intraarterial pressure rises.
Furthermore, the elastic modulus (Young’s modulus), E, is used in physics to define the elastic resistance of materials [9]. E is quantified as the association between the pressure applied and the distension achieved. To determine E of the aorta, the thickness of the arterial wall needs to be considered. In humans, we can derive the aortic E’ from the absolute change in pulse pressure, calculated as the difference between systolic and diastolic blood pressure (AP), linked to the related alterations in volume (AV): E’ = AP/AV [3]. Compliance (C) can be considered as the volume distensibility of a vessel, and is the reciprocal of elastic resistance: C = 1/E’ = AV/AP. The elasticity of the vessel wall is impaired when the intravascular pressure is increased and/or when the arterial stiffness intensifies with the aging of the individual [10].
4. Degenerative Changes in Large Arteries
A change in the aortic elastic properties is registered with human aging [11]. Being the largest artery in the organism, the aorta is the most susceptible to pathological stiffening after being cumulative exposed to CV risk factors (Figure 1). The ascending aorta has been proven to be the most elastic segment of the vessel. Therefore, it displays the earliest alterations with aging [12]. The main determinant for the increasing aortic stiffness is the medial layer of the aortic wall [13]. This vascular damage provokes vessel wall inflammation, followed by elastin degeneration and collagen deposition. Genetics play a significant role in the reduction of aortic elasticity. Previously published data revealed that gene modifications in the matrix metalloprotein-9 gene are independent predictors of reduced aortic elasticity [14]. Furthermore, inherited elastic tissue diseases, such as Marfan and Ehlers–Danlos syndrome, are related to increased aortic wall stiffness from early age [15]. On the other hand, there is an abundance of data demonstrating the relation between diabetes and vessel stiffness [16][17]. Patients with reduced kidney function are characterized with dysregulated mineral metabolism. An enhanced inflammatory biomarkers’ synthesis—such as C-reactive protein, tumor necrosis factor (TNF)α, and interleukin (IL-)6—has been registered [18]. Moreover, patients with chronic kidney disease are more prone to electrolyte disbalance, which could lead to increased arterial stress [19]. On the other hand, in the case of chronic aortic regurgitation, arterial compliance and distensibility are aggravated. The latter phenomenon is probably a result of a compensatory mechanism to counteract the extensive stroke of the heart [20]. The absence of elevated arterial compliance is linked to rapid hemodynamic deterioration and disease progression [21].
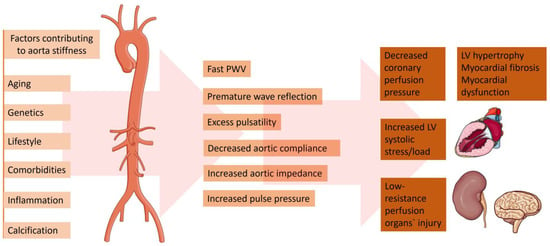
Figure 1. Factors contributing to the increased aorta stiffness and pathophysiological and clinical consequences. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 5 February 2024).
5. Methods for Evaluation Arterial Elastic Properties
5.1. Pulse Wave Velocity
Evaluation of arterial pulsed wave velocity is one of the most widespread approaches for the noninvasive evaluation of arterial stiffness. Oscillometric pulse wave velocity (o-PWV) emerges as an attractive, operator-independent, and non-invasive technique for assessing arterial stiffness. It leverages the measurement of arterial pressure oscillations to offer valuable insights into arterial rigidity, contributing a vital component to cardiovascular risk assessment.
After it has been proven as a simple and accurate method, to predict adverse CV outcomes [26][27][28], the latest clinical recommendations have included the measurement of PWV as the gold standard method for screening and CV risk stratification in patients with arterial hypertension [29]. Pulse wave velocity may be obtained by acquiring the transit time of the pulse from the pressure waveforms at two different sites of a current vascular segment. Carotid–femoral pulse wave velocity (CFPWV) represents a global estimate of the arterial stiffness through the entire aorta and, as such, it is a currently widely accepted method for evaluating aortic elasticity [30]. This method is easily applied because of the superficial site of the common carotid and femoral arteries. However, the measurement of arterial pulse waveforms may be technically cumbersome in patients who are overweight. Another technique for assessing arterial elasticity is the cardiac–ankle vascular index (CAVI). To determine the CAVI, phonocardiography data together with brachial and ankle pulse waveforms are required [31]. The heart-to-ankle transit time is determined as the period between the pulse onset at the heart and the upstroke of the ankle pulse waveform. A probable pitfall is the insertion of a long muscular arterial segment (femoral to ankle), which may lead to inaccuracies. Although easily applicable and reproducible, the method of PWV is a surrogate marker, which integrates an estimate of aortic elasticity along the entire vessel length. Therefore, it does not offer data on segmental aortic stiffness. Previous data have revealed that separate vessel segments have different compliance and do not stiffen uniformly. Hence, the difference in the elastic properties of the distinct vessel segments may have an important impact on the overall cardiovascular risk [32].
5.2. Echocardiography
Transthoracic echocardiography is an omnipresent method, widely applicable in current clinical practice. Aortic elasticity may be determined by obtaining vessel diameters or cross-sectional area in systole and diastole [32]. On the other hand, transesophageal echocardiography (TOE) is a more specialized imaging modality requiring more experience. Aortic measurements obtained from TOE may be more accurate due to the proximity of the esophagus and the aorta [33].
5.3. Magnetic Resonance Imaging
With the technology advent in recent years, cardiac magnetic resonance imaging has enabled us to obtain accurate vessel pulse wave velocity. The method allows us to acquire wave velocity simultaneously at two or more arterial locations. Furthermore, the distance between the two vessel segments can be measured with high accuracy without any approximation [34].
5.4. Computed Tomography Angiography
In recent decades, the improvement of technology has made the acquisition of computed tomography cardiac images possible with high temporal resolution [33][34][35]. Previously published data revealed that arterial elasticity measurements acquired using electrocardiographically gated CT were feasible in phantom, porcine specimens, and aortic models of polydimethylsiloxane [36][37]. Advantages of computed tomography over other imaging modalities are its high spatial resolution and capability to characterize atherosclerotic plaque. Furthermore, CT allows us to compare regional aortic elasticity [38]. A previous study revealed a negative association between age and ascending aortic elasticity by using electrocardiographically (ECG) gated dual-source (DS) CT [39]. It is worth mentioning that the protocols for the evaluation of aortic elastic properties used in the various studies with CT are different and not standardized. In some studies, to calculate the aortic distensibility, a cross-sectional area (CSA) was measured by paralleling the body axis [40][41][42][43]. On the other hand, others obtained CSA perpendicular to the aortic center line [39]. There is no comparison of the different methods to understand which technique provides the most accurate measurements, Figure 2.
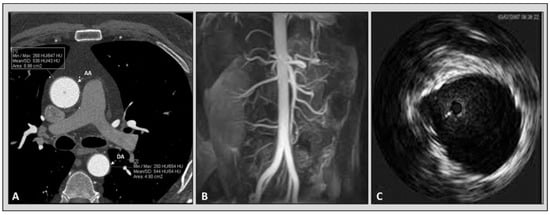
Figure 2. Imaging modalities available for the evaluation of aortic elastic properties. Panel (A): computed tomography angiography. Panel (B): magnetic resonance angiography. Panel (C): intravascular ultrasound imaging of aorta.
5.5. Intravascular Ultrasound Imaging of the Aorta
The intravascular ultrasound (IVUS) was used for the first time in 1990 by Weintraub to evaluate a patient with aortic dissection [44]. Afterwards, there have been a few reports and published studies considering the use of IVUS for the imaging of aortic pathology [45][46][47]. In 1985, Hughes et al. published data about an in vivo experiment with dogs, analyzing the application of intravascular ultrasonic catheter with a simultaneous evaluation of intra-aortic pressure [48]. This study revealed that the acquisition of arterial dimensions with this ultrasonic system may lay the foundations of a new method for aortic elastic properties evaluation. Almost 10 years later, another group performed a study assessing the aortic distensibility in six patients using intravascular ultrasound imaging [49]. It was shown that IVUS may provide an accurate in vivo evaluation of human aortic compliance. In a small study including 12 patients with enlarged ascending aorta who underwent both CT angiography and IVUS, researchers did not find a significant difference between the measurements and aortic elastic parameters among the two modalities [50].
References
- El-Naggar, H.M.; Anwar, H.S.; Helmy, H.; Demitry, S.R. Aortic Elasticity Indices as Predictors of Coronary Artery Disease Severity Assessed by SYNTAX Score. J. Cardiovasc. Echography 2021, 31, 234–241.
- Cengiz Elçioglu, B.; Kılıç, A.; Baydar, O.; Şahin, Ş.T.; Çelik, H.G.; Aytekin, V.; Aytekin, S. Evaluation of Aortic Elasticity Parameters Measured by Transthoracic Echocardiography in a Normotensive Population: A Single-Center Study. Normotansif Bir Popülasyonda Aortik Elastikiyet Parametrelerinin Transtorasik Ekokardiyografi ile Değerlendirilmesi: Tek Merkezli Bir Çalışma. Turk Kardiyol. Dern. Ars. Turk Kardiyol. Derneginin Yayin. Organidir 2023, 51, 369–377.
- Kassab, G.S. Biomechanics of the cardiovascular system: The aorta as an illustratory example. J. R. Soc. Interface 2006, 3, 719–740.
- Bader, H. biochemistry. Importance of the gerontology of elastic arteries in the development of essential hypertension. Clin. Physiol. Biochem. 1983, 1, 36–56.
- Mohiaddin, R.H.; Underwood, S.R.; Bogren, H.G.; Firmin, D.N.; Klipstein, R.H.; Rees, R.S.; Longmore, D.B. Regional aortic compliance studied by magnetic resonance imaging: The effects of age, training, and coronary artery disease. Heart 1989, 62, 90–96.
- Belz, G.G. Elastic properties and Windkessel function of the human aorta. Cardiovasc. Drugs Ther. 1995, 9, 73–83.
- Nichols, W.; O’Rourke, M.; Kenney, W.L. McDonald’s Blood Flow in Arteries: Theoretical, Experimental and Clinical Principles, 3rd. ed.; LWW: Philadelphia, PA, USA, 1991.
- O’Rourke, M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990, 15, 339–347.
- Roberts, R.J.; Rowe, R.C.; York, P. The relationship between Young’s modulus of elasticity of organic solids and their molecular structure. Powder Technol. 1991, 65, 139–146.
- Stratos, C.; Stefanadis, C.; Kallikazaros, I.; Boudoulas, H.; Toutouzas, P. Ascending aorta distensibility abnormalities in hypertensive patients and response to nifedipine administration. Am. J. Med. 1992, 93, 505–512.
- Angoff, R.; Mosarla, R.C.; Tsao, C.W. Aortic stiffness: Epidemiology, risk factors, and relevant biomarkers. Front. Cardiovasc. Med. 2021, 8, 709396.
- Redheuil, A.; Yu, W.C.; Wu, C.O.; Mousseaux, E.; De Cesare, A.; Yan, R.; Kachenoura, N.; Bluemke, D.; Lima, J.A. Reduced ascending aortic strain and distensibility: Earliest manifestations of vascular aging in humans. Hypertension 2010, 55, 319–326.
- Cavalcante, J.L.; Lima, J.A.; Redheuil, A.; Al-Mallah, M.H. Aortic stiffness: Current understanding and future directions. J. Am. Coll. Cardiol. 2011, 57, 1511–1522.
- Yasmin; McEniery, C.M.; O’Shaughnessy, K.M.; Harnett, P.; Arshad, A.; Wallace, S.; Maki-Petaja, K.; McDonnell, B.; Ashby, M.J.; Brown, J.; et al. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 1799–1805.
- Harada, K.; Yasuoka, K.; Shimada, Y. Usefulness of tissue doppler imaging for assessing aortic wall stiffness in children with the marfan syndrome. Am. J. Cardiol. 2004, 93, 1072–1075.
- Lee, J.M.; Shirodaria, C.; E Jackson, C.; Robson, M.D.; Antoniades, C.; Francis, J.M.; Wiesmann, F.; Channon, K.M.; Neubauer, S.; Choudhury, R.P. Multi-modal magnetic resonance imaging quantifies atherosclerosis and vascular dysfunction in patients with type 2 diabetes mellitus. Diabetes Vasc. Dis. Res. 2007, 4, 44–48.
- Stacey, R.B.; Bertoni, A.G.; Eng, J.; Bluemke, D.A.; Hundley, W.G.; Herrington, D. Modification of the effect of glycemic status on aortic distensibility by age in the multi-ethnic study of atherosclerosis. Hypertension 2010, 55, 26–32.
- Safar, M.E.; London, G.M.; Plante, G.E. Arterial stiffness and kidney function. Hypertension 2004, 43, 163–168.
- Doyle, A.; Mark, P.B.; Johnston, N.; Foster, J.; Connell, J.M.; Dargie, H.; Jardine, A.; Padmanabhan, N. Aortic stiffness and diastolic flow abnormalities in end-stage renal disease assessed by magnetic resonance imaging. Nephron Clin. Prac. 2008, 109, c1–c8.
- Kopel, L.; Tarasoutchi, F.; Medeiros, C.; Carvalho, R.T.; Grinberg, M.; Lage, S.G. Arterial distensibility as a possible compensatory mechanism in chronic aortic regurgitation. Arq. Bras. Cardiol. 2001, 77, 262–265.
- Wilson, R.A.; McDonald, R.W.; Bristow, J.; Cheitlin, M.; Nauman, D.; Massie, B.; Greenberg, B. Correlates of aortic distensibility in chronic aortic regurgitation and relation to progression to surgery. J. Am. Coll. Cardiol. 1992, 19, 733–738.
- Roccabianca, S.; Figueroa, C.; Tellides, G.; Humphrey, J. Quantification of regional differences in aortic stiffness in the aging human. J. Mech. Behav. Biomed. Mater. 2014, 29, 618–634.
- Segers, P.; De Backer, J.; Devos, D.; Rabben, S.I.; Gillebert, T.C.; Van Bortel, L.M.; De Sutter, J.; De Paepe, A.; Verdonck, P.R. Aortic reflection coefficients and their association with global indexes of wave reflection in healthy controls and patients with Marfan’s syndrome. Am. J. Physiol.-Heart Circ. Physiol. 2006, 290, H2385–H2392.
- Lind, L.; Fors, N.; Hall, J.; Marttala, K.; Stenborg, A. A comparison of three different methods to determine arterial compliance in the elderly: The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study. J. Hypertens. 2006, 24, 1075–1082.
- Tanriverdi, H.; Evrengul, H.; Kara, C.O.; Kuru, O.; Tanriverdi, S.; Ozkurt, S.; Kaftan, A.; Kilic, M. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: Noninvasive indicators of atherosclerosis. Respiration 2006, 73, 741–750.
- Jae, S.Y.; Heffernan, K.S.; Park, J.B.; Kurl, S.; Kunutsor, S.K.; Kim, J.-Y.; A Laukkanen, J. Association between estimated pulse wave velocity and the risk of cardiovascular outcomes in men. Eur. J. Prev. Cardiol. 2021, 28, e25–e27.
- Garcia-Carretero, R.; Vigil-Medina, L.; Barquero-Perez, O.; Ramos-Lopez, J. Pulse wave velocity and machine learning to predict cardiovascular outcomes in prediabetic and diabetic populations. J. Med. Syst. 2020, 44, 16.
- Vlachopoulos, C.; Aznaouridis, K.; Stefanadis, C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010, 55, 1318–1327.
- Williams, B.; Mancia, G.; Spiering, W.; Rosei, E.A.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; De Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur. Heart J. 2018, 39, 3021–3104.
- Park, J.B.; Sharman, J.E.; Li, Y.; Munakata, M.; Shirai, K.; Chen, C.-H.; Jae, S.Y.; Tomiyama, H.; Kosuge, H.; Bruno, R.M.; et al. Expert Consensus on the Clinical Use of Pulse Wave Velocity in Asia. Pulse 2022, 10, 1–18.
- Hayashi, K.; Yamamoto, T.; Takahara, A.; Shirai, K. Clinical assessment of arterial stiffness with cardio-ankle vascular index: Theory and applications. J. Hypertens. 2015, 33, 1742–1757.
- Nelson, A.J.; Worthley, S.G.; Cameron, J.D.; Willoughby, S.R.; Piantadosi, C.; Carbone, A.; Dundon, B.K.; Leung, M.C.; A Hope, S.; Meredith, I.T.; et al. Cardiovascular magnetic resonance-derived aortic distensibility: Validation and observed regional differences in the elderly. J. Hypertens. 2009, 27, 535–542.
- SCOT-Heart Investigators. Coronary CT angiography and 5-year risk of myocardial infarction. N. Engl. J. Med. 2018, 379, 924–933.
- Taguchi, K.; Anno, H. High temporal resolution for multislice helical computed tomography. Med. Phys. 2000, 27, 861–872.
- Soschynski, M.; Hagen, F.; Baumann, S.; Hagar, M.T.; Weiss, J.; Krauss, T.; Schlett, C.L.; Mühlen, C.v.Z.; Bamberg, F.; Nikolaou, K.; et al. High temporal resolution dual-source photon-counting CT for coronary artery disease: Initial multicenter clinical Experience. J. Clin. Med. 2022, 11, 6003.
- Ganten, M.-K.; Krautter, U.; von Tengg-Kobligk, H.; Böckler, D.; Schumacher, H.; Stiller, W.; Delorme, S.; Kauczor, H.-U.; Kauffmann, G.W.; Bock, M. Quantification of aortic distensibility in abdominal aortic aneurysm using ECG-gated multi-detector computed tomography. Eur. Radiol. 2008, 18, 966–973.
- Medynsky, A.; Sherebrin, M.; Rankin, R.; Holdsworth, D.; Roach, M. (Eds.) An in vitro time study of distensibility in porcine aortas using high resolution X-ray CT. In Proceedings of the 1996 Fifteenth Southern Biomedical Engineering Conference, Dayton, OH, USA, 29–31 March 1996; IEEE: Piscataway, NJ, USA, 1996.
- Carrascosa, P.; Capuñay, C.; Deviggiano, A.; Rodríguez-Granillo, G.A.; Sagarduy, M.I.; Cortines, P.; Carrascosa, J.; Parodi, J.C. Thoracic aorta cardiac-cycle related dynamic changes assessed with a 256-slice CT scanner. Cardiovasc. Diagn. Ther. 2013, 3, 125–128.
- Li, N.; Beck, T.; Chen, J.; Biermann, C.; Guo, L.; Sun, H.; Gao, F.; Liu, C. Assessment of thoracic aortic elasticity: A preliminary study using electrocardiographically gated dual-source CT. Eur. Radiol. 2011, 21, 1564–1572.
- Ahmadi, N.; Nabavi, V.; Hajsadeghi, F.; Flores, F.; Azmoon, S.; Ismaeel, H.; Shavelle, D.; Mao, S.S.; Ebrahimi, R.; Budoff, M.J. Impaired aortic distensibility measured by computed tomography is associated with the severity of coronary artery disease. Int. J. Cardiovasc. Imaging 2011, 27, 459–469.
- Ganten, M.; Krautter, U.; Hosch, W.; Hansmann, J.; von Tengg-Kobligk, H.; Delorme, S.; Kauczor, H.-U.; Kauffmann, G.W.; Bock, M. Age related changes of human aortic distensibility: Evaluation with ECG-gated CT. Eur. Radiol. 2007, 17, 701–708.
- Okuyama, T.; Ehara, S.; Shirai, N.; Sugioka, K.; Yamashita, H.; Kataoka, T.; Naruko, T.; Itoh, T.; Otani, K.; Matsuoka, T.; et al. Assessment of aortic atheromatous plaque and stiffness by 64-slice computed tomography is useful for identifying patients with coronary artery disease. Circ. J. 2008, 72, 2021–2027.
- Jang, S.; Yong, H.S.; Doo, K.W.; Kang, E.-Y.; Woo, O.H.; Choi, E.J. Relation of aortic calcification, wall thickness, and distensibility with severity of coronary artery disease: Evaluation with coronary CT angiography. Acta Radiol. 2012, 53, 839–844.
- Weintraub, A.R.; Schwartz, S.L.; Pandian, N.G.; E Katz, S.; Kwon, O.J.; Millan, V.; Bojar, R. Evaluation of acute aortic dissection by intravascular ultrasonography. N. Engl. J. Med. 1990, 323, 1566–1567.
- Alfonso, F.; Goicolea, J.; Aragoncillo, P.; Hernandez, R.; Macaya, C. Diagnosis of aortic intramural hematoma by intravascular ultrasound imaging. Am. J. Cardiol. 1995, 76, 735–738.
- Mileva, N.; Vassilev, D.; Gil, R.; Rigatelli, G. Misdiagnosed aortic intramural hematoma and the role of intravascular ultrasound imaging in detection of acute aortic syndrome: A Case Report. Cardiovasc. Innov. Appl. 2018, 2, 447–449.
- Wei, H.; Schiele, F.; Meneveau, N.; Seronde, M.-F.; Legalery, P.; Caulfield, F.; Bonneville, J.-F.; Chocron, S.; Bassand, J.-P. The value of intravascular ultrasound imaging in diagnosis of aortic penetrating atherosclerotic ulcer. EuroIntervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2006, 1, 432–437.
- Hughes, D.J.; Fearnot, N.E.; Babbs, C.F.; Bourland, J.D.; Geddes, L.A.; Eggelton, R. Continuous measurement of aortic radius change in vivo with an intra-aortic ultrasonic catheter. Med. Biol. Eng. Comput. 1985, 23, 197–202.
- Hansen, M.E.; Yucel, E.K.; Megerman, J.; L’Italien, G.J.; Abbott, W.M.; Waltman, A.C. In vivo determination of human arterial compliance: Preliminary investigation of a new technique. Cardiovasc. Interv. Radiol. 1994, 17, 22–26.
- Mileva, N.B.; Vassilev, D.I. Intravascular Ultrasound Imaging for Evaluation of Aortic Elastic Properties: Review of the Literature and a Single-Center Experience. Cardiol. Cardiovasc. Med. 2020, 4, 396–399.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
559
Revisions:
2 times
(View History)
Update Date:
08 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




