Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Tsvetelina Velikova and Version 2 by Sirius Huang.
Cardiovascular risk stratification is a cornerstone of preventive cardiology, aiming to identify individuals at a higher risk for adverse events. In line with this, aortic elastic properties have gained recognition as crucial indicators of vascular health and predictors of cardiovascular outcomes.
- aortic elasticity
- stiffness
- compliance
- cardiovascular risk
- vascular health
- atherosclerosis
1. Introduction
The assessment of aortic elastic properties stands at the intersection of cardiovascular research, diagnosis, and risk stratification. The aorta, a vital conduit of the circulatory system, is heavily burdened in maintaining pulsatile blood flow, buffering cardiac pulsations, and reducing afterload on the left ventricle. During each cardiac cycle, the left ventricle (LV) ejects blood into the aorta, generating a high-pressure wave. However, the pulse wave is not directly transmitted to the smaller vasculature but is initially transformed in the aorta. The elastic properties of the large arteries enable the expansion of the vessel in order to accommodate the stroke volume and attenuate e the high-pressure pulsations. This aortic distention protects the smaller arteries and capillaries from the pulsatile pressure that may injure their fragile structure.
Therefore, disturbances in aortic elastic properties, characterized by stiffness, compliance, and distensibility changes, have been considered as important factors in the pathogenesis of numerous cardiovascular pathologies [1][2][1,2]. These comprise various disorders such as arterial hypertension, atherosclerosis, acute and chronic aortic syndromes, and valvular heart diseases [1]. If the aortic elasticity is compromised, it loses its ability to diminish the high pulsatile pressure developed in the left ventricle, therefore exposing the cardiovascular system to heightened stress.
2. Physiology of Large Arteries
One of the main characteristics of the elastic arteries is that their medial layer is rich in collagen and elastin filaments. This quality enables the stretch in response to ventricular contractions [3][6]. It has been revealed that the thoracic aorta contains up to 40% elastic filaments. However, the amount of elastin decreases with the reduction of the vessel size in peripheral circulation [4][7]. Reduced elasticity has been proved in the segments of the aorta from the proximal to the distal vessel parts [5][8]. The elastic arteries during systole store almost half of the LV stroke volume. In contrast, in diastole, the aortic wall elastic forces release this residual volume to the periphery vessels continuously, ensuring a stable peripheral circulation. This hemodynamic process has been described as the Windkessel effect [6][9]. During LV systole, the heart ejects about 60–100 mL of blood into the aorta and arteries, with relatively 50% of the stroke volume being forwarded directly to peripheral circulation. However, the aorta acts as an elastic reservoir, storing the other 50% of the stroke volume [4][7]. With the fall in aortic pressure in diastole, the aorta recoils gradually, and the stored blood volume is ejected into peripheral circulation. Therefore, pressure and blood flow are maintained throughout diastole, and a relatively continuous peripheral flow is provided despite the pulsatile myocardial contractions. Thus, the elastic properties of the large arteries enable the sustained pressure in the arteries despite the alternating left ventricular ejection and the pulsatile blood flow. Apart from peripheral circulation, this physiologic process also affects the heart, by reducing the LV afterload, which leads to an improvement in coronary blood flow and left ventricular relaxation [7][10]. One other mechanism necessary for maintaining adequate diastolic pressure is that, usually, in a healthy organism, the pulse wave velocity in the large arteries is rather slow. The reflection of this signal in peripheral circulation, and the return of the wave to the ascending aorta forms the dicrotic wave during early diastole [8][11]. The Windkessel effect depends on the elastic properties of the aorta [6][9]. The physics definition of elastic materials are substances that readopt their initial shape after the application of an external force. During a myocardial contraction, the ejected stroke volume kinetic energy is first converted into potential energy within the ballooned aortic wall [6][9]. Afterwards, the stored potential energy is transformed into kinetic energy during diastole when the aorta gradually recoils [7][10]. Therefore, despite the diastolic cessation of the heart’s contraction, the amount of blood within the peripheral arteries does not reach a diastolic halt, and blood pressure does not decrease to null.3. Concept of Elasticity, Stiffness, and Compliance
The aortic elastic properties include both the ability to distend due to the increased pressure and the ability to recoil gradually to the initial vessel size as the blood pressure reduces in diastole. The vessels’ elastic resistance (elastance) explains the resistance in which the large arteries push against their distention in a situation where an additional volume is ejected and when the intraarterial pressure rises. Furthermore, the elastic modulus (Young’s modulus), E, is used in physics to define the elastic resistance of materials [9][12]. E is quantified as the association between the pressure applied and the distension achieved. To determine E of the aorta, the thickness of the arterial wall needs to be considered. In humans, we can derive the aortic E’ from the absolute change in pulse pressure, calculated as the difference between systolic and diastolic blood pressure (AP), linked to the related alterations in volume (AV): E’ = AP/AV [3][6]. Compliance (C) can be considered as the volume distensibility of a vessel, and is the reciprocal of elastic resistance: C = 1/E’ = AV/AP. The elasticity of the vessel wall is impaired when the intravascular pressure is increased and/or when the arterial stiffness intensifies with the aging of the individual [10][13].4. Degenerative Changes in Large Arteries
A change in the aortic elastic properties is registered with human aging [11][14]. Being the largest artery in the organism, the aorta is the most susceptible to pathological stiffening after being cumulative exposed to CV risk factors (Figure 1). The ascending aorta has been proven to be the most elastic segment of the vessel. Therefore, it displays the earliest alterations with aging [12][15]. The main determinant for the increasing aortic stiffness is the medial layer of the aortic wall [13][16]. This vascular damage provokes vessel wall inflammation, followed by elastin degeneration and collagen deposition. Genetics play a significant role in the reduction of aortic elasticity. Previously published data revealed that gene modifications in the matrix metalloprotein-9 gene are independent predictors of reduced aortic elasticity [14][17]. Furthermore, inherited elastic tissue diseases, such as Marfan and Ehlers–Danlos syndrome, are related to increased aortic wall stiffness from early age [15][18]. On the other hand, there is an abundance of data demonstrating the relation between diabetes and vessel stiffness [16][17][19,20]. Patients with reduced kidney function are characterized with dysregulated mineral metabolism. An enhanced inflammatory biomarkers’ synthesis—such as C-reactive protein, tumor necrosis factor (TNF)α, and interleukin (IL-)6—has been registered [18][21]. Moreover, patients with chronic kidney disease are more prone to electrolyte disbalance, which could lead to increased arterial stress [19][22]. On the other hand, in the case of chronic aortic regurgitation, arterial compliance and distensibility are aggravated. The latter phenomenon is probably a result of a compensatory mechanism to counteract the extensive stroke of the heart [20][23]. The absence of elevated arterial compliance is linked to rapid hemodynamic deterioration and disease progression [21][24].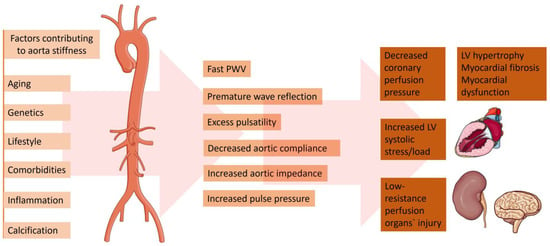
Figure 1. Factors contributing to the increased aorta stiffness and pathophysiological and clinical consequences. Parts of the figure were drawn by using pictures from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/, accessed on 5 February 2024).
5. Methods for Evaluation Arterial Elastic Properties
Several different methods to assess arterial compliance have been documented [22][23][24][25][25,26,27,28].5.1. Pulse Wave Velocity
Evaluation of arterial pulsed wave velocity is one of the most widespread approaches for the noninvasive evaluation of arterial stiffness. Oscillometric pulse wave velocity (o-PWV) emerges as an attractive, operator-independent, and non-invasive technique for assessing arterial stiffness. It leverages the measurement of arterial pressure oscillations to offer valuable insights into arterial rigidity, contributing a vital component to cardiovascular risk assessment. After it has been proven as a simple and accurate method, to predict adverse CV outcomes [26][27][28][29,30,31], the latest clinical recommendations have included the measurement of PWV as the gold standard method for screening and CV risk stratification in patients with arterial hypertension [29][32]. Pulse wave velocity may be obtained by acquiring the transit time of the pulse from the pressure waveforms at two different sites of a current vascular segment. Carotid–femoral pulse wave velocity (CFPWV) represents a global estimate of the arterial stiffness through the entire aorta and, as such, it is a currently widely accepted method for evaluating aortic elasticity [30][33]. This method is easily applied because of the superficial site of the common carotid and femoral arteries. However, the measurement of arterial pulse waveforms may be technically cumbersome in patients who are overweight. Another technique for assessing arterial elasticity is the cardiac–ankle vascular index (CAVI). To determine the CAVI, phonocardiography data together with brachial and ankle pulse waveforms are required [31][34]. The heart-to-ankle transit time is determined as the period between the pulse onset at the heart and the upstroke of the ankle pulse waveform. A probable pitfall is the insertion of a long muscular arterial segment (femoral to ankle), which may lead to inaccuracies. Although easily applicable and reproducible, the method of PWV is a surrogate marker, which integrates an estimate of aortic elasticity along the entire vessel length. Therefore, it does not offer data on segmental aortic stiffness. Previous data have revealed that separate vessel segments have different compliance and do not stiffen uniformly. Hence, the difference in the elastic properties of the distinct vessel segments may have an important impact on the overall cardiovascular risk [32][35].5.2. Echocardiography
Transthoracic echocardiography is an omnipresent method, widely applicable in current clinical practice. Aortic elasticity may be determined by obtaining vessel diameters or cross-sectional area in systole and diastole [32][35]. On the other hand, transesophageal echocardiography (TOE) is a more specialized imaging modality requiring more experience. Aortic measurements obtained from TOE may be more accurate due to the proximity of the esophagus and the aorta [33][36].5.3. Magnetic Resonance Imaging
With the technology advent in recent years, cardiac magnetic resonance imaging has enabled us to obtain accurate vessel pulse wave velocity. The method allows us to acquire wave velocity simultaneously at two or more arterial locations. Furthermore, the distance between the two vessel segments can be measured with high accuracy without any approximation [34][37].5.4. Computed Tomography Angiography
In recent decades, the improvement of technology has made the acquisition of computed tomography cardiac images possible with high temporal resolution [33][34][35][36,37,38]. Previously published data revealed that arterial elasticity measurements acquired using electrocardiographically gated CT were feasible in phantom, porcine specimens, and aortic models of polydimethylsiloxane [36][37][39,40]. Advantages of computed tomography over other imaging modalities are its high spatial resolution and capability to characterize atherosclerotic plaque. Furthermore, CT allows us to compare regional aortic elasticity [38][41]. A previous study revealed a negative association between age and ascending aortic elasticity by using electrocardiographically (ECG) gated dual-source (DS) CT [39][42]. It is worth mentioning that the protocols for the evaluation of aortic elastic properties used in the various studies with CT are different and not standardized. In some studies, to calculate the aortic distensibility, a cross-sectional area (CSA) was measured by paralleling the body axis [40][41][42][43][43,44,45,46]. On the other hand, others obtained CSA perpendicular to the aortic center line [39][42]. There is no comparison of the different methods to understand which technique provides the most accurate measurements, Figure 23.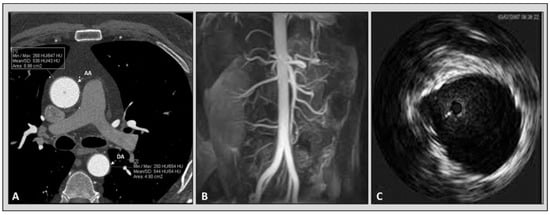
Figure 23. Imaging modalities available for the evaluation of aortic elastic properties. Panel (A): computed tomography angiography. Panel (B): magnetic resonance angiography. Panel (C): intravascular ultrasound imaging of aorta.
