Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Mitsuhiro Fujishiro | -- | 1119 | 2024-03-07 09:24:55 | | | |
| 2 | Fanny Huang | Meta information modification | 1119 | 2024-03-11 06:45:22 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Fujishiro, M. Endoscopic Diagnosis of Early Gastric Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/55957 (accessed on 08 February 2026).
Fujishiro M. Endoscopic Diagnosis of Early Gastric Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/55957. Accessed February 08, 2026.
Fujishiro, Mitsuhiro. "Endoscopic Diagnosis of Early Gastric Cancer" Encyclopedia, https://encyclopedia.pub/entry/55957 (accessed February 08, 2026).
Fujishiro, M. (2024, March 07). Endoscopic Diagnosis of Early Gastric Cancer. In Encyclopedia. https://encyclopedia.pub/entry/55957
Fujishiro, Mitsuhiro. "Endoscopic Diagnosis of Early Gastric Cancer." Encyclopedia. Web. 07 March, 2024.
Copy Citation
Endoscopy is mandatory to detect early gastric cancer (EGC). When considering the cost-effectiveness of the endoscopic screening of EGC, risk stratification by combining serum pepsinogen values and anti-H. pylori IgG antibody values is very promising. After the detection of suspicious lesions of EGC, a detailed observation using magnifying endoscopy with band-limited light is necessary, which reveals an irregular microsurface and/or an irregular microvascular pattern with demarcation lines in the case of cancerous lesions. Endocytoscopy enables us to make an in vivo histological diagnosis.
early gastric cancer
endoscopic diagnosis
1. Introduction
Although the number of patients with gastric cancer is expected to decrease in the future due to the decline in the number of people infected with Helicobacter pylori (H. pylori) and the spread of H. pylori eradication therapy, the incidence of gastric cancer still ranks fifth among all cancer types (1,089,103), and it caused the fourth largest number of deaths (768,793) in 2020 in the world [1]. With the spread of esophagogastroduodenoscopy (EGD) in daily practice, the early detection of gastric cancer became possible. Further combined with the birth of endoscopic submucosal dissection (ESD) in the late 1990s [2][3], the era in which early-stage gastric cancer without lymph node metastasis can be cured with endoluminal surgery alone has arrived.
2. The Role of Endoscopy in Gastric Cancer Detection
Japan is one of the few countries where population-based mass screening of gastric cancer has been carried out for a long time. X-ray examination with barium meal had been the only recommended mass screening method until 2016; however, it has been fraught with various problems, such as a low participation rate (less than 50% of target generations) and radiation exposure. In the 2014 edition of the “Gastric Cancer Screening Guidelines Based on Efficacy Evaluation” published by the National Cancer Center in Japan, endoscopy was recommended for population-based mass screening for the first time, along with X-ray examination, because there is sufficient evidence that it has a mortality reduction effect [4][5].
According to the Screening Guidelines, endoscopy once every 2–3 years is recommended for individuals over 50 years old who have an increased risk of gastric cancer. However, it is not practical to perform EGD on every individual over the age of 50 in terms of cost-effectiveness and endoscopist manpower. Since the H. pylori infection is involved in the development of gastric cancer in more than 95% of cases, and it is known that the risk of developing gastric cancer increases with the progression of gastric mucosa atrophy and intestinal metaplasia due to H. pylori infection, stratified screening according to the risk of gastric cancer should be explored.
The screening of gastric cancer using the ABC classification is a method that stratifies the risk of developing gastric cancer by combining serum pepsinogen (PG) values, which are serum markers of atrophic gastritis, and anti-H. pylori IgG antibody (HP) values [6]. In a previous report, where PGI ≤ 70 ng/mL and PGI/II ratio ≤ 3.0 were defined as PG-positive (with atrophic gastritis), the risk of developing gastric cancer in Group A (HP-negative PG-negative), Group B (HP-positive PG-negative), Group C (HP-positive PG-positive), and Group D (HP-negative PG-positive) was reported to be 0.016%, 0.14%, 0.30%, and 1.1%, respectively [7] (Figure 1). Based on this report, it has been proposed that Group A should not undergo endoscopy (or it should be conducted once every 5 years), Group B should be examined once every 3 years, Group C should be examined once every 2 years, and group D should be examined annually.
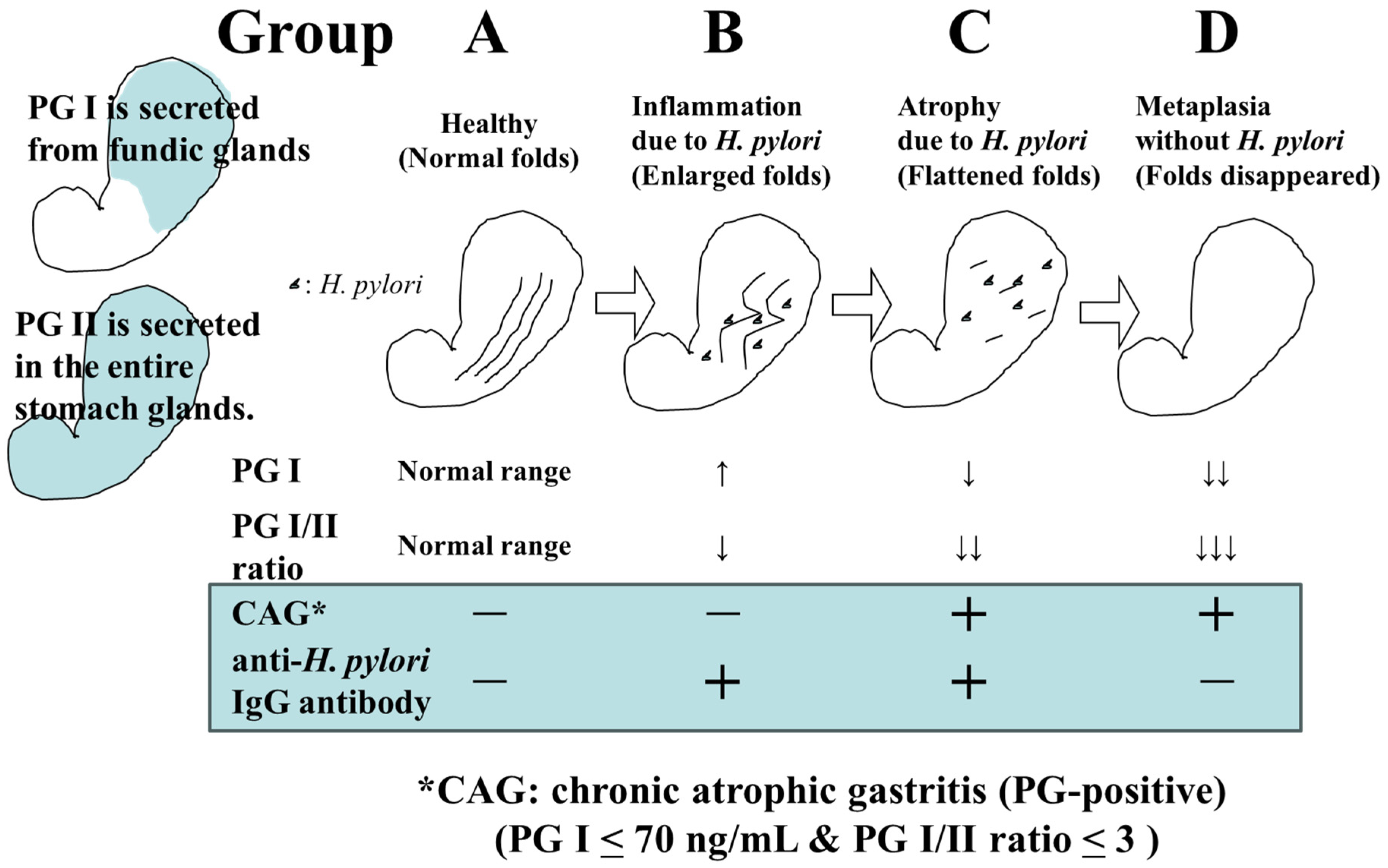
Figure 1. Changes in H. pylori infection status, gastric environment, and serum PG and HP levels.
However, with the spread of H. pylori eradication therapy, there may be more than a few cases of H. pylori eradication in Group A; therefore, it is not appropriate to uniformly withhold endoscopy from Group A. It is necessary to take relevant measures such as considering the history of H. pylori eradication and performing endoscopy once every 2–3 years for eradication cases. A subgroup analysis has also pointed out that Group B includes patients with a high incidence of gastric cancer (e.g., PGI > 70 ng/mL and PGI/II ratio ≤ 3.0); thus, more optimal risk stratification and appropriate endoscopic intervals should be explored through further examination [8].
When a patient is presumed to be uninfected with H. pylori, based on endoscopic findings indicating no atrophy and the existence of regular arrangements of collecting venules (RAC) in the gastric angle [9], the risk of cancer is extremely low and thus endoscopy can be completed in a short time. However, regarding an H. pylori-infected or -eradicated patient, when it is determined that the risk of cancer is high based on the endoscopic findings such as the progression of open-type atrophy, the presence of intestinal metaplasia, enlarged folds of the gastric body, and nodular gastritis in the gastric antrum, a more detailed examination is required. The latter two findings are important in classifying patients at high risk of undifferentiated gastric cancer [10][11].
3. Endoscopic Diagnosis of Early Gastric Cancer
Elevated and protruded cancers, which are principally characterized as areas with irregularities and a whitish color, are usually picked up by white light observation. When the mucosal surface structure is different from the surroundings, differentiation has to be made as to whether such lesions are non-neoplastic lesions, such as fundus gland polyps, hyperplastic polyps, and intestinal metaplasia, or neoplastic lesions, such as adenomas and cancer. Those with remarkable irregularities, large nodules, and noticeable redness in some parts, and that are >2 cm in size are likely to be cancer.
Flat and depressed cancers, which are characterized as areas with irregularities (a stellate shape) and a reddish or whitish color, are usually picked up by white light observation. Although it is often difficult to distinguish between cancer and non-cancer, new technologies are helping to overcome such difficulties. Detailed observation using magnifying endoscopy with band-limited light, such as narrow-band imaging (NBI), reveals an irregular microsurface and/or an irregular microvascular pattern with demarcation lines in the case of cancerous lesions (Figure 2). This new algorithm is known as the magnifying endoscopy simple diagnostic algorithm for gastric cancer (MESDA-G) [12], and it is based on the vessels plus surface (VS) classification system [13]. Although the progress of endoscopic diagnostic technology has been remarkable as described above, histological diagnosis with biopsy has to be conducted to confirm whether a lesion is cancer.
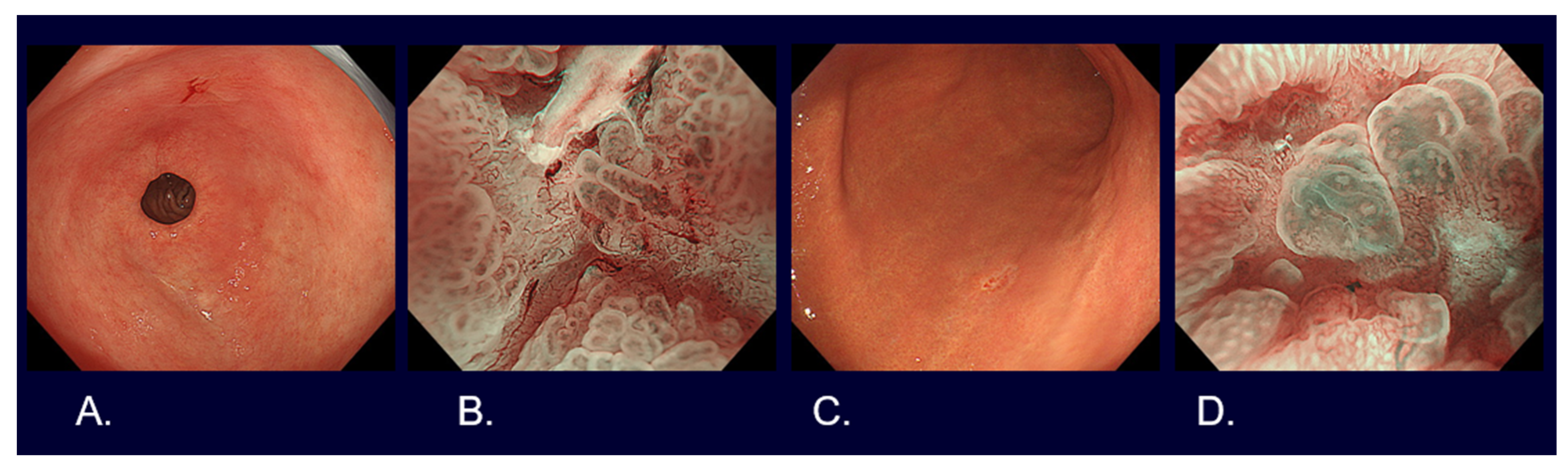
Figure 2. Endoscopic images of early gastric cancers. (A) White light observation of type 0-IIc differentiated cT1a without ulcerative findings, <2 cm in size, on the lesser curve of the gastric antrum. (B) Narrow-band imaging observation with 80 times magnification of Figure 1A lesion (irregular microvascular pattern with demarcation line). (C) White light observation of type 0-IIc undifferentiated cT1a without ulcerative findings, <2 cm in size, on the greater curve of the gastric body. (D) Narrow-band imaging observation with 80 times magnification of Figure 1C lesion (irregular microvascular pattern with demarcation line).
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249.
- Kato, M. Endoscopic Submucosal Dissection (ESD) Is Being Accepted as a New Procedure of Endoscopic Treatment of Early Gastric Cancer. Intern. Med. Tokyo Jpn. 2005, 44, 85–86.
- Ono, H.; Kondo, H.; Gotoda, T.; Shirao, K.; Yamaguchi, H.; Saito, D.; Hosokawa, K.; Shimoda, T.; Yoshida, S. Endoscopic Mucosal Resection for Treatment of Early Gastric Cancer. Gut 2001, 48, 225–229.
- Hamashima, C. Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines Update Version of the Japanese Guidelines for Gastric Cancer Screening. Jpn. J. Clin. Oncol. 2018, 48, 673–683.
- Mabe, K.; Inoue, K.; Kamada, T.; Kato, K.; Kato, M.; Haruma, K. Endoscopic Screening for Gastric Cancer in Japan: Current Status and Future Perspectives. Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2022, 34, 412–419.
- Miki, K. Gastric Cancer Screening by Combined Assay for Serum Anti-Helicobacter Pylori IgG Antibody and Serum Pepsinogen Levels-“ABC Method”. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 405–414.
- Yoshida, T.; Kato, J.; Inoue, I.; Yoshimura, N.; Deguchi, H.; Mukoubayashi, C.; Oka, M.; Watanabe, M.; Enomoto, S.; Niwa, T.; et al. Cancer Development Based on Chronic Active Gastritis and Resulting Gastric Atrophy as Assessed by Serum Levels of Pepsinogen and Helicobacter Pylori Antibody Titer. Int. J. Cancer 2014, 134, 1445–1457.
- Watanabe, M.; Kato, J.; Inoue, I.; Yoshimura, N.; Yoshida, T.; Mukoubayashi, C.; Deguchi, H.; Enomoto, S.; Ueda, K.; Maekita, T.; et al. Development of Gastric Cancer in Nonatrophic Stomach with Highly Active Inflammation Identified by Serum Levels of Pepsinogen and Helicobacter Pylori Antibody Together with Endoscopic Rugal Hyperplastic Gastritis. Int. J. Cancer 2012, 131, 2632–2642.
- Yagi, K.; Nakamura, A.; Sekine, A. Characteristic Endoscopic and Magnified Endoscopic Findings in the Normal Stomach without Helicobacter Pylori Infection. J. Gastroenterol. Hepatol. 2002, 17, 39–45.
- Nishibayashi, H.; Kanayama, S.; Kiyohara, T.; Yamamoto, K.; Miyazaki, Y.; Yasunaga, Y.; Shinomura, Y.; Takeshita, T.; Takeuchi, T.; Morimoto, K.; et al. Helicobacter Pylori-Induced Enlarged-Fold Gastritis Is Associated with Increased Mutagenicity of Gastric Juice, Increased Oxidative DNA Damage, and an Increased Risk of Gastric Carcinoma. J. Gastroenterol. Hepatol. 2003, 18, 1384–1391.
- Kamada, T.; Tanaka, A.; Yamanaka, Y.; Manabe, N.; Kusunoki, H.; Miyamoto, M.; Tanaka, S.; Hata, J.; Chayama, K.; Haruma, K. Nodular Gastritis with Helicobacter pylori Infection Is Strongly Associated with Diffuse-Type Gastric Cancer in Young Patients. Dig. Endosc. 2007, 19, 180–184.
- Muto, M.; Yao, K.; Kaise, M.; Kato, M.; Uedo, N.; Yagi, K.; Tajiri, H. Magnifying Endoscopy Simple Diagnostic Algorithm for Early Gastric Cancer (MESDA-G). Dig. Endosc. Off. J. Jpn. Gastroenterol. Endosc. Soc. 2016, 28, 379–393.
- Yao, K.; Anagnostopoulos, G.K.; Ragunath, K. Magnifying Endoscopy for Diagnosing and Delineating Early Gastric Cancer. Endoscopy 2009, 41, 462–467.
More
Information
Subjects:
Gastroenterology & Hepatology
Contributor
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
614
Revisions:
2 times
(View History)
Update Date:
11 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




