Endoscopy is mandatory to detect early gastric cancer (EGC). When considering the cost-effectiveness of the endoscopic screening of EGC, risk stratification by combining serum pepsinogen values and anti-H. pylori IgG antibody values is very promising. After the detection of suspicious lesions of EGC, a detailed observation using magnifying endoscopy with band-limited light is necessary, which reveals an irregular microsurface and/or an irregular microvascular pattern with demarcation lines in the case of cancerous lesions. Endocytoscopy enables us to make an in vivo histological diagnosis.
1. Introduction
Although the number of patients with gastric cancer is expected to decrease in the future due to the decline in the number of people infected with
Helicobacter pylori (
H. pylori) and the spread of
H. pylori eradication therapy, the incidence of gastric cancer still ranks fifth among all cancer types (1,089,103), and it caused the fourth largest number of deaths (768,793) in 2020 in the world [
1]. With the spread of esophagogastroduodenoscopy (EGD) in daily practice, the early detection of gastric cancer became possible. Further combined with the birth of endoscopic submucosal dissection (ESD) in the late 1990s [
2,
3], the era in which early-stage gastric cancer without lymph node metastasis can be cured with endoluminal surgery alone has arrived.
2. The Role of Endoscopy in Gastric Cancer Detection
Japan is one of the few countries where population-based mass screening of gastric cancer has been carried out for a long time. X-ray examination with barium meal had been the only recommended mass screening method until 2016; however, it has been fraught with various problems, such as a low participation rate (less than 50% of target generations) and radiation exposure. In the 2014 edition of the “Gastric Cancer Screening Guidelines Based on Efficacy Evaluation” published by the National Cancer Center in Japan, endoscopy was recommended for population-based mass screening for the first time, along with X-ray examination, because there is sufficient evidence that it has a mortality reduction effect [
4,
5].
According to the Screening Guidelines, endoscopy once every 2–3 years is recommended for individuals over 50 years old who have an increased risk of gastric cancer. However, it is not practical to perform EGD on every individual over the age of 50 in terms of cost-effectiveness and endoscopist manpower. Since the H. pylori infection is involved in the development of gastric cancer in more than 95% of cases, and it is known that the risk of developing gastric cancer increases with the progression of gastric mucosa atrophy and intestinal metaplasia due to H. pylori infection, stratified screening according to the risk of gastric cancer should be explored.
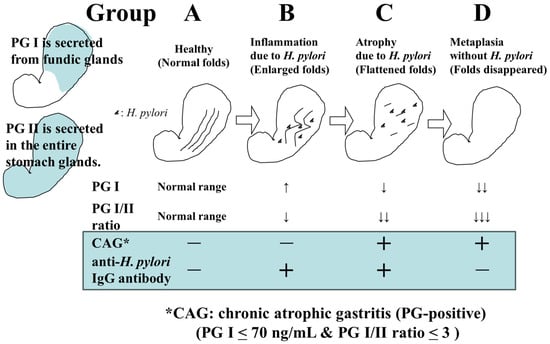
The screening of gastric cancer using the ABC classification is a method that stratifies the risk of developing gastric cancer by combining serum pepsinogen (PG) values, which are serum markers of atrophic gastritis, and anti-
H. pylori IgG antibody (HP) values [
6]. In a previous report, where PGI ≤ 70 ng/mL and PGI/II ratio ≤ 3.0 were defined as PG-positive (with atrophic gastritis), the risk of developing gastric cancer in Group A (HP-negative PG-negative), Group B (HP-positive PG-negative), Group C (HP-positive PG-positive), and Group D (HP-negative PG-positive) was reported to be 0.016%, 0.14%, 0.30%, and 1.1%, respectively [
7] (
Figure 1). Based on this report, it has been proposed that Group A should not undergo endoscopy (or it should be conducted once every 5 years), Group B should be examined once every 3 years, Group C should be examined once every 2 years, and group D should be examined annually.
Figure 1. Changes in H. pylori infection status, gastric environment, and serum PG and HP levels.
However, with the spread of
H. pylori eradication therapy, there may be more than a few cases of
H. pylori eradication in Group A; therefore, it is not appropriate to uniformly withhold endoscopy from Group A. It is necessary to take relevant measures such as considering the history of
H. pylori eradication and performing endoscopy once every 2–3 years for eradication cases. A subgroup analysis has also pointed out that Group B includes patients with a high incidence of gastric cancer (e.g., PGI > 70 ng/mL and PGI/II ratio ≤ 3.0); thus, more optimal risk stratification and appropriate endoscopic intervals should be explored through further examination [
8].
When a patient is presumed to be uninfected with
H. pylori, based on endoscopic findings indicating no atrophy and the existence of regular arrangements of collecting venules (RAC) in the gastric angle [
9], the risk of cancer is extremely low and thus endoscopy can be completed in a short time. However, regarding an
H. pylori-infected or -eradicated patient, when it is determined that the risk of cancer is high based on the endoscopic findings such as the progression of open-type atrophy, the presence of intestinal metaplasia, enlarged folds of the gastric body, and nodular gastritis in the gastric antrum, a more detailed examination is required. The latter two findings are important in classifying patients at high risk of undifferentiated gastric cancer [
10,
11].
3. Endoscopic Diagnosis of Early Gastric Cancer
Elevated and protruded cancers, which are principally characterized as areas with irregularities and a whitish color, are usually picked up by white light observation. When the mucosal surface structure is different from the surroundings, differentiation has to be made as to whether such lesions are non-neoplastic lesions, such as fundus gland polyps, hyperplastic polyps, and intestinal metaplasia, or neoplastic lesions, such as adenomas and cancer. Those with remarkable irregularities, large nodules, and noticeable redness in some parts, and that are >2 cm in size are likely to be cancer.
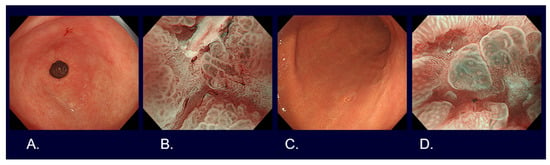
Flat and depressed cancers, which are characterized as areas with irregularities (a stellate shape) and a reddish or whitish color, are usually picked up by white light observation. Although it is often difficult to distinguish between cancer and non-cancer, new technologies are helping to overcome such difficulties. Detailed observation using magnifying endoscopy with band-limited light, such as narrow-band imaging (NBI), reveals an irregular microsurface and/or an irregular microvascular pattern with demarcation lines in the case of cancerous lesions (
Figure 2). This new algorithm is known as the magnifying endoscopy simple diagnostic algorithm for gastric cancer (MESDA-G) [
12], and it is based on the vessels plus surface (VS) classification system [
13]. Although the progress of endoscopic diagnostic technology has been remarkable as described above, histological diagnosis with biopsy has to be conducted to confirm whether a lesion is cancer.
Figure 2. Endoscopic images of early gastric cancers. (A) White light observation of type 0-IIc differentiated cT1a without ulcerative findings, <2 cm in size, on the lesser curve of the gastric antrum. (B) Narrow-band imaging observation with 80 times magnification of Figure 1A lesion (irregular microvascular pattern with demarcation line). (C) White light observation of type 0-IIc undifferentiated cT1a without ulcerative findings, <2 cm in size, on the greater curve of the gastric body. (D) Narrow-band imaging observation with 80 times magnification of Figure 1C lesion (irregular microvascular pattern with demarcation line).
This entry is adapted from the peer-reviewed paper 10.3390/cancers16051039