
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sumit Kumar | -- | 4874 | 2024-03-06 08:41:03 | | | |
| 2 | Jessie Wu | + 6 word(s) | 4880 | 2024-03-06 09:54:53 | | | | |
| 3 | Rajesh Raghupathy | Meta information modification | 4891 | 2024-03-08 20:43:49 | | | | |
| 4 | Rajesh Raghupathy | Meta information modification | 4891 | 2024-03-08 20:45:59 | | | | |
| 5 | Jessie Wu | Meta information modification | 4891 | 2024-03-11 01:05:21 | | | | |
| 6 | Rajesh Raghupathy | + 75 word(s) | 4966 | 2024-03-11 09:35:05 | | | | |
| 7 | Rajesh Raghupathy | -56 word(s) | 4910 | 2024-03-12 08:19:36 | | | | |
| 8 | Jessie Wu | Meta information modification | 4910 | 2024-03-12 08:26:52 | | |
Video Upload Options
Lithium-based electrolytes are, at least from a thermodynamic standpoint, the most suitable ion-transport materials for energy storage systems. However, lithium-based ionic conductors suffer from safety concerns, and the limited availability of lithium in the Earth’s crust is at the root of the need to consider alternative metal ions. Notably, sodium stands out as the sixth most-prevalent element; therefore, when considering mineral reserves, it as a very attractive candidate as an alternative to the status quo. Even if the specific energy and energy density of sodium are indeed inferior with respect to those of lithium, there is substantial economic appeal in promoting the use of the former metal in stationary energy storage applications.
1. Solid Polymers Electrolytes (SPEs)
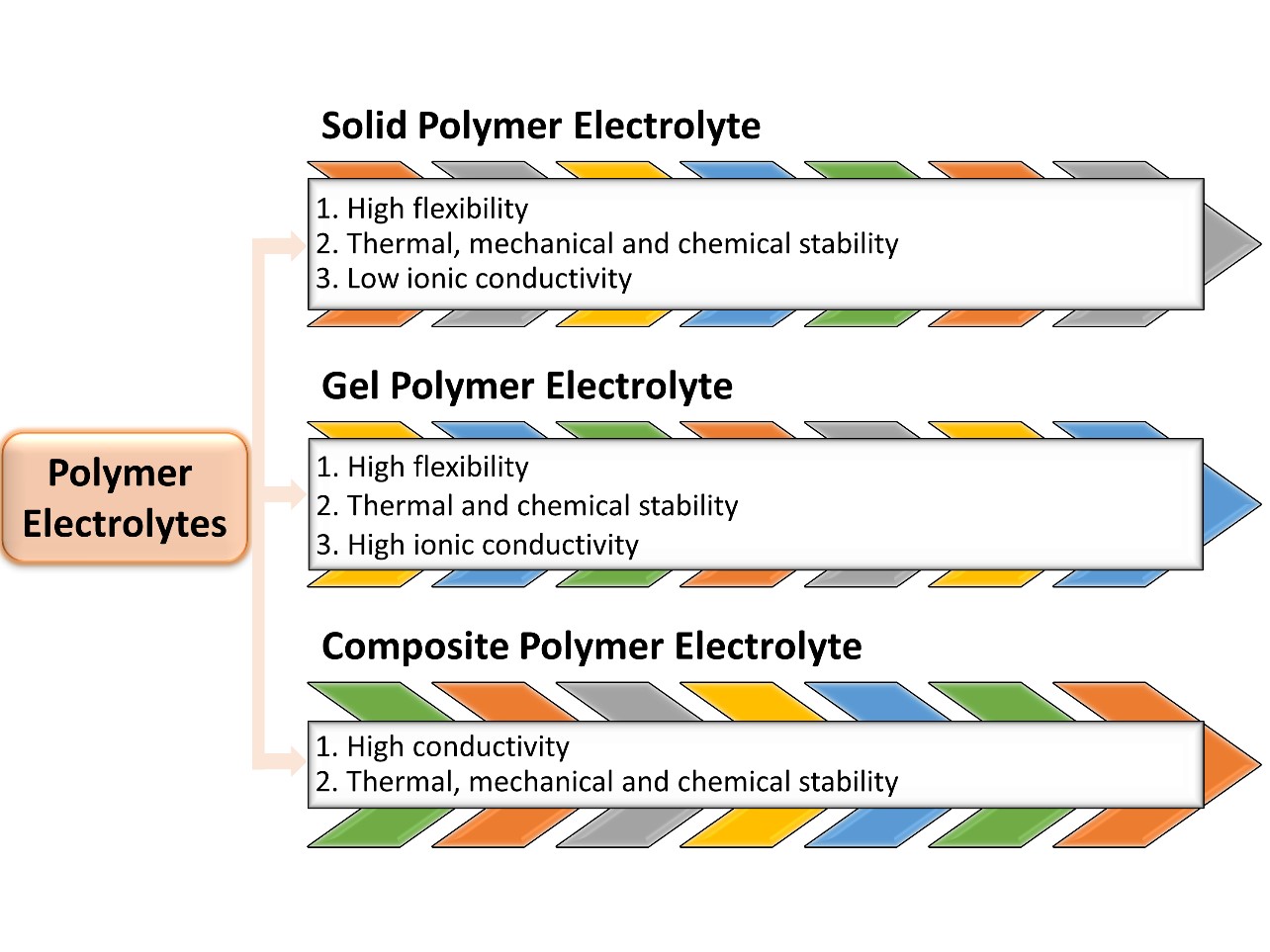
2. Gel Polymers Electrolytes (GPEs)
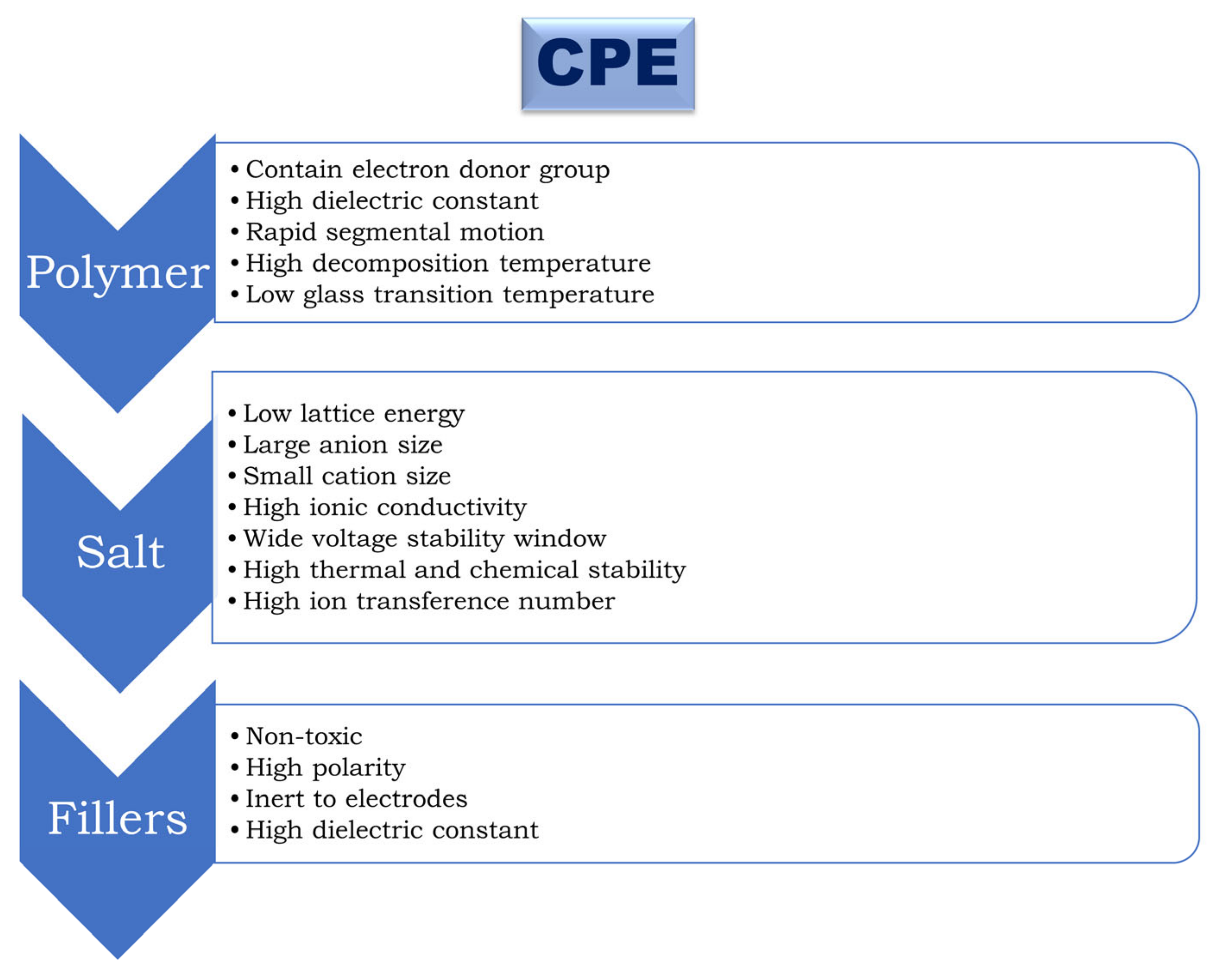
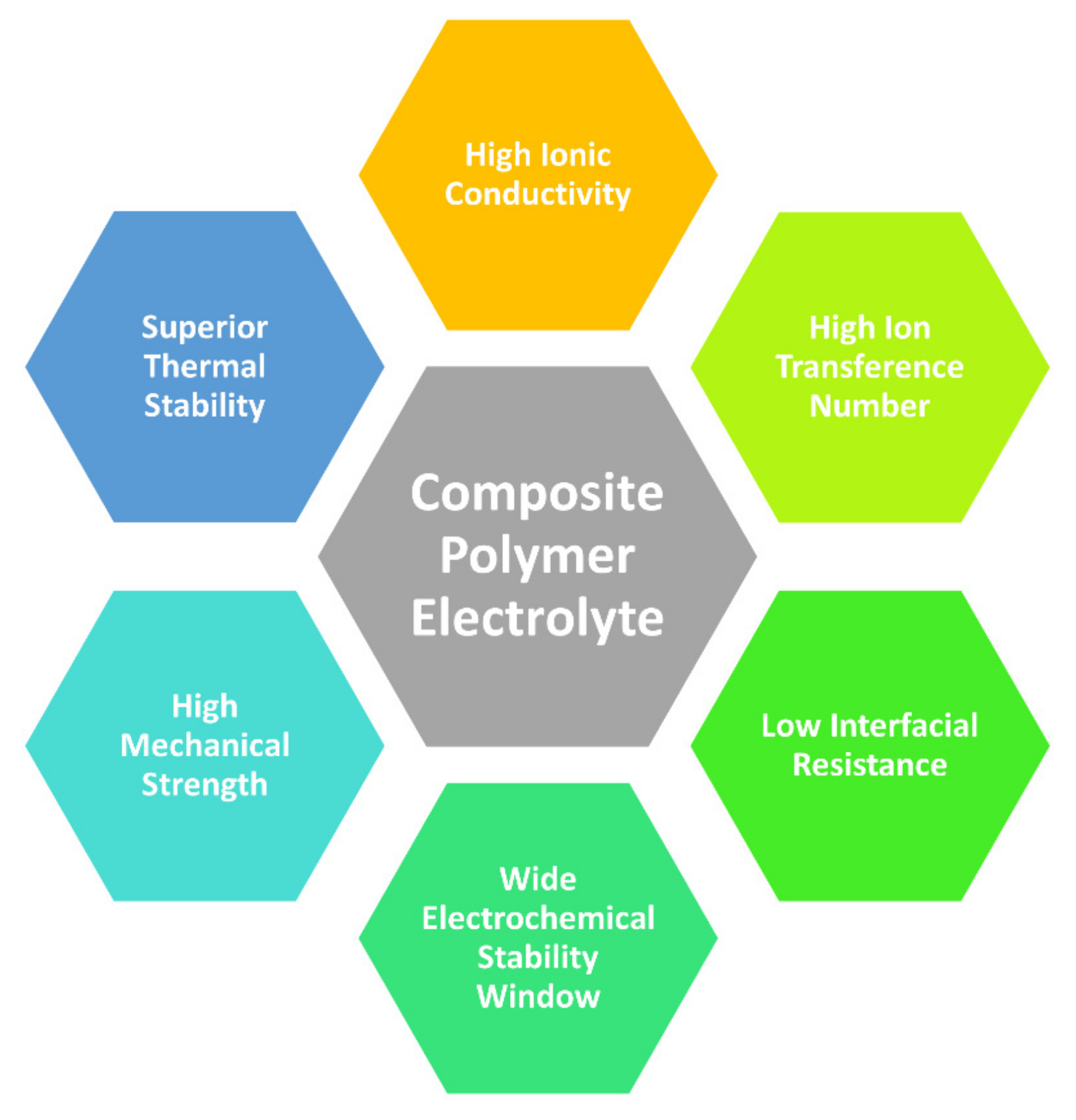
3. Composite Polymer Electrolytes (CPEs)
References
- Tiwari, T.; Srivastava, N.; Srivastava, P.C. Ion Dynamics Study of Potato Starch + Sodium Salts Electrolyte System. Int. J. Electrochem. 2013, 2013, 670914.
- Aragón, M.J.; Gutiérrez, J.; Klee, R.; Lavela, P.; Alcántara, R.; Tirado, J.L. On the effect of carbon content for achieving a high performing Na3V2(PO4)3/C nanocomposite as cathode for sodium-ion batteries. J. Electroanal. Chem. 2017, 784, 47–54.
- Aziz, S.B.; Brevik, I.; Hamsan, M.H.; Brza, M.A.; Nofal, M.M.; Abdullah, A.M.; Rostam, S.; Al-Zangana, S.; Muzakir, S.K.; Kadir, M.F.Z. Compatible Solid Polymer Electrolyte Based on Methyl Cellulose for Energy Storage Application: Structural, Electrical, and Electrochemical Properties. Polymers 2020, 12, 2257.
- Gao, H.; Zhou, W.; Park, K.; Goodenough, J.B. A Sodium-Ion Battery with a Low-Cost Cross-Linked Gel-Polymer Electrolyte. Adv. Energy Mater. 2016, 6, 1600467.
- Wang, Y.; Song, S.; Xu, C.; Hu, N.; Molenda, J.; Lu, L. Development of solid-state electrolytes for sodium-ion battery–A short review. Nano Mater. Sci. 2019, 1, 91–100.
- Xu, G.-L.; Amine, R.; Abouimrane, A.; Che, H.; Dahbi, M.; Ma, Z.-F.; Saadoune, I.; Alami, J.; Mattis, W.L.; Pan, F. Challenges in Developing Electrodes, Electrolytes, and Diagnostics Tools to Understand and Advance Sodium-Ion Batteries. Adv. Energy Mater. 2018, 8, 1702403.
- Moon, S.-H.; Kim, Y.H.; Cho, D.-C.; Shin, E.-C.; Lee, D.; Im, W.B.; Lee, J.-S. Sodium ion transport in polymorphic scandium NASICON analog Na3Sc2(PO4)3 with new dielectric spectroscopy approach for current-constriction effects. Solid State Ion. 2016, 289, 55–71.
- Anantha, P.S.; Hariharan, K. Physical and ionic transport studies on poly(ethylene oxide)–NaNO3 polymer electrolyte system. Solid State Ion. 2005, 176, 155–162.
- Hashmi, S.A.; Chandra, S. Experimental investigations on a sodium-ion-conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. Mater. Sci. Eng. B 1995, 34, 18–26.
- Sreekanth, T.; Jaipal Reddy, M.; Ramalingaiah, S.; Subba Rao, U.V. Ion-conducting polymer electrolyte based on poly (ethylene oxide) complexed with NaNO3 salt-application as an electrochemical cell. J. Power Sources 1999, 79, 105–110.
- Chandrasekaran, R.; Selladurai, S. Preparation and characterization of a new polymer electrolyte (PEO:NaClO3) for battery application. J. Solid State Electrochem. 2001, 5, 355–361.
- Siva Kumar, J.; Subrahmanyam, A.R.; Jaipal Reddy, M.; Subba Rao, U.V. Preparation and study of properties of polymer electrolyte system (PEO+NaClO3). Mater. Lett. 2006, 60, 3346–3349.
- Mohan, V.M.; Raja, V.; Sharma, A.K.; Narasimha Rao, V.V.R. Ion transport and battery discharge characteristics of polymer electrolyte based on PEO complexed with NaFeF4 salt. Ionics 2006, 12, 219–226.
- Mohan, V.M.; Bhargav, P.B.; Raja, V.; Sharma, A.K.; Narasimha Rao, V.V.R. Optical and Electrical Properties of Pure and Doped PEO Polymer Electrolyte Films. Soft Mater. 2007, 5, 33–46.
- Bhide, A.; Hariharan, K. Ionic transport studies on (PEO)6:NaPO3 polymer electrolyte plasticized with PEG400. Eur. Polym. J. 2007, 43, 4253–4270.
- Bhide, A.; Hariharan, K. A new polymer electrolyte system (PEO)n:NaPO3. J. Power Sources 2006, 159, 1450–1457.
- Sasikala, U.T.; Kumar, P.N.; Rao, V.V.R.N.; Sharma, A.K. Structural, Electrical and Parametric studies of a PEO based Polymer Electrolyte for battery Applications. Int. J. Eng. Sci. Adv. Technol. 2012, 2, 722–730.
- Ma, Q.; Liu, J.; Qi, X.; Rong, X.; Shao, Y.; Feng, W.; Nie, J.; Hu, Y.-S.; Li, H.; Huang, X. A new Na-based polymer electrolyte for solid-state sodium batteries. J. Mater. Chem. A 2017, 5, 7738–7743.
- Herath, H.M.A.; Seneviratne, V.A. Electrical and Thermal studies on Sodium based polymer electrolyte. Procedia Eng. 2017, 215, 124–129.
- Arya, A.; Sharma, A.L. Tailoring of the structural, morphological, electrochemical, and dielectric properties of solid polymer electrolyte. Ionics 2019, 25, 1617–1632.
- Pritam; Arya, A.; Sharma, A.L. Dielectric relaxations and transport properties parameter analysis of novel blended solid polymer electrolyte for sodium-ion rechargeable batteries. J. Mater. Sci. 2019, 54, 7131–7155.
- Pritam; Arya, A.; Sharma, A.L. Selection of best composition of Na+ ion conducting PEO-PEI blend solid polymer electrolyte based on structural, electrical, and dielectric spectroscopic analysis. Ionics 2020, 26, 745–766.
- Zhang, Q.; Lu, Y.; Yu, H.; Yang, G.; Liu, Q.; Wang, Z.; Chen, L.; Hu, Y.-S. PEO-NaPF6 Blended Polymer Electrolyte for Solid State Sodium Battery. J. Electrochem. Soc. 2020, 167, 070523.
- Youcef, H.B.; Orayech, B.; Del Amo, J.M.L.; Bonilla, F.; Shanmukaraj, D.; Armand, M. Functionalized cellulose as quasi single-ion conductors in polymer electrolyte for all-solid–state Li/Na and LiS batteries. Solid State Ion. 2020, 345, 115168.
- Kim, Y.; Künzel, M.; Steinle, D.; Dong, X.; Kim, G.-T.; Varzi, A.; Passerini, S. Anode-less seawater batteries with a Na-ion conducting solid-polymer electrolyte for power to metal and metal to power energy storage. Energy Environ. Sci. 2022, 15, 2610–2618.
- Chandra, A.; Chandra, A.; Dhundhel, R.S.; Bhatt, A. Sodium-ion-conducting solid polymer electrolyte: Temperature-dependent ionic parameters and solid-state polymer battery fabrication. Indian J. Phys. 2022, 96, 1069–1074.
- Subba Reddy, C.V.; Han, X.; Zhu, Q.-Y.; Mai, L.-Q.; Chen, W. Conductivity and discharge characteristics of (PVC+NaClO4) polymer electrolyte systems. Eur. Polym. J. 2006, 42, 3114–3120.
- Muhammad, F.H.; Subban, R.H.Y.; Winie, T. Charge carrier density and mobility of poly(vinyl chloride)-based polymer electrolyte using impedance spectroscopy. Mater. Today Proc. 2017, 4 Pt C, 5130–5137.
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V.V.R.N. Structural and electrical studies of sodium iodide doped poly(vinyl alcohol) polymer electrolyte films for their application in electrochemical cells. Ionics 2007, 13, 173–178.
- Bhargav, P.B.; Mohan, V.M.; Sharma, A.K.; Rao, V.V.R.N. Structural and electrical properties of pure and NaBr doped poly (vinyl alcohol) (PVA) polymer electrolyte films for solid-state battery applications. Ionics 2007, 13, 441–446.
- Duraikkan, V.; Sultan, A.B.; Nallaperumal, N.; Shunmuganarayanan, A. Structural, thermal and electrical properties of polyvinyl alcohol/poly(vinyl pyrrolidone)–sodium nitrate solid polymer blend electrolyte. Ionics 2018, 24, 139–151.
- Aziz, S.B.; Nofal, M.M.; Abdulwahid, R.T.; Ghareeb, H.O.; Dannoun, E.M.A.; Abdullah, R.M.; Hamsan, M.H.; Kadir, M.F.Z. Plasticized Sodium-Ion Conducting PVA Based Polymer Electrolyte for Electrochemical Energy Storage—EEC Modeling, Transport Properties, and Charge-Discharge Characteristics. Polymers 2021, 13, 803.
- Khanmirzaei, M.H.; Ramesh, S.; Ramesh, K. Polymer electrolyte based dye-sensitized solar cell with rice starch and 1-methyl-3-propylimidazolium iodide ionic liquid. Mater. Des. 2015, 85, 833–837.
- Binti Shahrudin, S.; Ahmad, A.H. Electrical Analysis of Cornstarch-Based Polymer Electrolyte Doped with NaCl. Solid State Phenom. 2017, 268, 347–351.
- Awang, F.; Hassan, M.; Kamarudin, K. Effect of Sodiumbisulfite on corn starch solid polymer electrolyte. Malays. J. Anal. Sci. 2021, 25, 224–233.
- Asnawi, A.S.F.M.; Aziz, S.B.; Brevik, I.; Brza, M.A.; Yusof, Y.M.; Alshehri, S.M.; Ahamad, T.; Kadir, M.F.Z. The Study of Plasticized Sodium-Ion Conducting Polymer Blend Electrolyte Membranes Based on Chitosan/Dextran Biopolymers: Ion Transport, Structural, Morphological and Potential Stability. Polymers 2021, 13, 383.
- Rani, N.S.; Sannappa, J.; Demappa, T.; Mahadevaiah, A. Structural, thermal, and electrical studies of sodium iodide (NaI)-doped hydroxypropyl methylcellulose (HPMC) polymer electrolyte films. Ionics 2014, 20, 201–207.
- Abiddin, J.F.B.; Ahmad, A. Fourier transform infrared spectroscopy and electrical characterization of methylcellulose based solid polymer electrolyte doped with sodium iodide. J. Teknol. 2015, 76, 41–45.
- Bella, F.; Colò, F.; Nair, J.R.; Gerbaldi, C. Photopolymer Electrolytes for Sustainable, Upscalable, Safe, and Ambient-Temperature Sodium-Ion Secondary Batteries. ChemSusChem 2015, 8, 3668–3676.
- El Sayed, A.M.; Khabiri, G. Spectroscopic, Optical and Dielectric Investigation of (Mg, Cu, Ni, or Cd) Acetates’ Influence on Carboxymethyl Cellulose Sodium Salt/Polyvinylpyrrolidone Polymer Electrolyte Films. J. Electron. Mater. 2020, 49, 2381–2392.
- Shetty, S.K.; Ismayil, N.; Shetty, G. Enhancement of Electrical and Optical Properties of Sodium Bromide Doped Carboxymethyl Cellulose Biopolymer Electrolyte Films. J. Macromol. Sci. Part B 2020, 59, 235–247.
- Singh, V.K.; Shalu; Chaurasia, S.K.; Singh, R.K. Development of ionic liquid mediated novel polymer electrolyte membranes for application in Na-ion batteries. RSC Adv. 2016, 6, 40199–40210.
- Zhang, J.; Wen, H.; Yue, L.; Chai, J.; Ma, J.; Hu, P.; Ding, G.; Wang, Q.; Liu, Z.; Cui, G. In Situ Formation of Polysulfonamide Supported Poly(ethylene glycol) Divinyl Ether Based Polymer Electrolyte toward Monolithic Sodium Ion Batteries. Small 2017, 13, 1601530.
- Pan, Q.; Li, Z.; Zhang, W.; Zeng, D.; Sun, Y.; Cheng, H. Single ion conducting sodium ion batteries enabled by a sodium ion exchanged poly(bis(4-carbonyl benzene sulfonyl)imide-co-2,5-diamino benzesulfonic acid) polymer electrolyte. Solid State Ion. 2017, 300, 60–66.
- Chen, S.; Feng, F.; Yin, Y.; Che, H.; Liao, X.-Z.; Ma, Z.-F. A solid polymer electrolyte based on star-like hyperbranched β-cyclodextrin for all-solid-state sodium batteries. J. Power Sources 2018, 399, 363–371.
- Faiz, H.; Zainuddin, S.K.; Kamarudin, K.; Kok Sheng, C.; Abdullah, M.A. Ion-conducting polymer electrolyte films based on poly (Sodium 4-styrenesulfonate) complexed with ammonium nitrate: Studies based on morphology, structural and electrical spectroscopy. Malays. J. Anal. Sci. 2018, 22, 238–248.
- Janakiraman, S.; Surendran, A.; Biswal, R.; Ghosh, S.; Anandhan, S.; Venimadhav, A. Electrospun electroactive polyvinylidene fluoride-based fibrous polymer electrolyte for sodium ion batteries. Mater. Res. Express 2019, 6, 086318.
- Yang, J.; Zhang, M.; Chen, Z.; Du, X.; Huang, S.; Tang, B.; Dong, T.; Wu, H.; Yu, Z.; Zhang, J. Flame-retardant quasi-solid polymer electrolyte enabling sodium metal batteries with highly safe characteristic and superior cycling stability. Nano Res. 2019, 12, 2230–2237.
- Liu, K.; Xie, Y.; Yang, Z.; Kim, H.-K.; Dzwiniel, T.L.; Yang, J.; Xiong, H.; Liao, C. Design of a Single-Ion Conducting Polymer Electrolyte for Sodium-Ion Batteries. J. Electrochem. Soc. 2021, 168, 120543.
- Law, H.M.; Yu, J.; Kwok, S.C.T.; Zhou, G.; Robson, M.J.; Wu, J.; Ciucci, F. A hybrid dual-salt polymer electrolyte for sodium metal batteries with stable room temperature cycling performance. Energy Storage Mater. 2022, 46, 182–191.
- Martinez-Cisneros, C.S.; Pandit, B.; Levenfeld, B.; Varez, A.; Sanchez, J.-Y. Flexible solvent-free polymer electrolytes for solid-state Na batteries. J. Power Sources 2023, 559, 232644.
- Olmedo-Martínez, J.L.; Fdz De Anastro, A.; Martínez-Ibañez, M.; Müller, A.J.; Mecerreyes, D. Polyethylene Oxide/Sodium Sulfonamide Polymethacrylate Blends as Highly Conducting Single-Ion Solid Polymer Electrolytes. Energy Fuels 2023, 37, 5519–5529.
- Cheng, X.; Pan, J.; Zhao, Y.; Liao, M.; Peng, H. Gel Polymer Electrolytes for Electrochemical Energy Storage. Adv. Energy Mater. 2018, 8, 1702184.
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, DONG, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui,, G. Safty -Enhanced Polymer Electrolytes for Sodium Batteries: Resent Progress and Perspectives.. ACS Appl. Mater. Interfaces. 2019, 11, 17109-17127.
- Feuillade, G.; Perche, P. Ion-conductive macromolecular gels and membranes for solid lithium cells. J. Appl. Electrochem. 1975, 5, 63–69.
- Baskoro, F.; Wong, H.Q.; Yen, H.-J. Strategic Structural Design of a Gel Polymer Electrolyte toward a High Efficiency Lithium-Ion Battery. ACS Appl. Energy Mater. 2019, 2, 3937–3971.
- Liang, S.; Yan, W.; Wu, X.; Zhang, Y.; Zhu, Y.; Wang, H.; Wu, Y. Gel polymer electrolytes for lithium ion batteries: Fabrication, characterization and performance. Solid State Ion. 2018, 318, 2–18.
- Hsueh, M.-F.; Huang, C.-W.; Wu, C.-A.; Kuo, P.-L.; Teng, H. The Synergistic Effect of Nitrile and Ether Functionalities for Gel Electrolytes Used in Supercapacitors. J. Phys. Chem. C 2013, 117, 16751–16758.
- Ostrovskii, D.; Brodin, A.; Torell, L.M.; Appetecchi, G.B.; Scrosati, B. Molecular and ionic interactions in poly(acrylonitrile)- and poly(methylmetacrylate)-based gel electrolytes. J. Chem. Phys. 1998, 109, 7618–7624.
- Zhu, M.; Wu, J.; Wang, Y.; Song, M.; Long, L.; Siyal, S.H.; Yang, X.; Sui, G. Recent advances in gel polymer electrolyte for high-performance lithium batteries. J. Energy Chem. 2019, 37, 126–142.
- Vincent, C.A. Applications of electroactive polymers. Edited by B. Scrosati. Chapman and Hall, London, 1993. pp. 354 price £40.00. ISBN 0-412-41430-9. Polym. Int. 1994, 33, 343.
- Sannier, L.; Bouchet, R.; Rosso, M.; Tarascon, J.M. Evaluation of GPE performances in lithium metal battery technology by means of simple polarization tests. J. Power Sources 2006, 158, 564–570.
- Groce, F.; Gerace, F.; Dautzemberg, G.; Passerini, S.; Appetecchi, G.B.; Scrosati, B. Synthesis and characterization of highly conducting gel electrolytes. Electrochim. Acta 1994, 39, 2187–2194.
- Hashmi, S.A.; Kumar, A.; Tripathi, S.K. Experimental studies on poly methyl methacrylate-based gel polymer electrolytes for application in electrical double layer capacitors. J. Phys. D Appl. Phys. 2007, 40, 6527.
- Michot, T.; Nishimoto, A.; Watanabe, M. Electrochemical properties of polymer gel electrolytes based on poly(vinylidene fluoride) copolymer and homopolymer. Electrochim. Acta 2000, 45, 1347–1360.
- Stallworth, P.E.; Greenbaum, S.G.; Croce, F.; Slane, S.; Salomon, M. Lithium-7 NMR and ionic conductivity studies of gel electrolytes based on poly(methylmethacrylate). Electrochim. Acta 1995, 40, 2137–2141.
- Karuppasamy, K.; Theerthagiri, J.; Vikraman, D.; Yim, C.-J.; Hussain, S.; Sharma, R.; Maiyalagan, T.; Qin, J.; Kim, H.-S. Ionic Liquid-Based Electrolytes for Energy Storage Devices: A Brief Review on Their Limits and Applications. Polymers 2020, 12, 918.
- Wang, X.; Liu, Z.; Wang, Y.; Chen, J.; Mao, Z.; Wang, D. Conductive Na2Zn2TeO6 Filler Modified Gel Polymer Electrolyte Membranes for Application in Sodium-Ions Batteries. ChemElectroChem 2020, 7, 5021–5028.
- Wang, P.; Zhang, H.; Chai, J.; Liu, T.; Hu, R.; Zhang, Z.; Li, G.; Cui, G. A novel single-ion conducting gel polymer electrolyte based on polymeric sodium tartaric acid borate for elevated-temperature sodium metal batteries. Solid State Ion. 2019, 337, 140–146.
- Wang, X.; Wang, X.; Chen, J.; Zhao, Y.; Mao, Z.; Wang, D. Durable sodium battery composed of conductive Ti3C2Tx MXene modified gel polymer electrolyte. Solid State Ion. 2021, 365, 115655.
- Vo, D.T.; Do, H.N.; Nguyen, T.T.; Nguyen, T.T.H.; Tran, V.M.; Okada, S.; Le, M.L.P. Sodium ion conducting gel polymer electrolyte using poly(vinylidene fluoride hexafluoropropylene). Mater. Sci. Eng. B 2019, 241, 27–35.
- Harshlata; Mishra, K.; Rai, D.K. Studies on ionic liquid-based nanocomposite gel polymer electrolyte and its application in sodium battery. Mater. Sci. Eng. B 2021, 267, 115098.
- Hwang, J.-Y.; Myung, S.-T.; Sun, Y.-K. Sodium-ion batteries: Present and future. Chem. Soc. Rev. 2017, 46, 3529–3614.
- Chen, G.; Zhang, K.; Liu, Y.; Ye, L.; Gao, Y.; Lin, W.; Xu, H.; Wang, X.; Bai, Y.; Wu, C. Flame-retardant gel polymer electrolyte and interface for quasi-solid-state sodium ion batteries. Chem. Eng. J. 2020, 401, 126065.
- Komaba, S.; Ishikawa, T.; Yabuuchi, N.; Murata, W.; Ito, A.; Ohsawa, Y. Fluorinated Ethylene Carbonate as Electrolyte Additive for Rechargeable Na Batteries. ACS Appl. Mater. Interfaces 2011, 3, 4165–4168.
- Shen, W.; Li, H.; Guo, Z.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Double-Nanocarbon Synergistically Modified Na3V2(PO4)3: An Advanced Cathode for High-Rate and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15341–15351.
- Yao, P.; Yu, H.; Ding, Z.; Liu, Y.; Lu, J.; Lavorgna, M.; Wu, J.; Liu, X. Review on Polymer-Based Composite Electrolytes for Lithium Batteries. Front. Chem. 2019, 7, 522.
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352.
- Yabuuchi, N.; Kubota, K.; Dahbi, M.; Komaba, S. Research Development on Sodium-Ion Batteries. Chem. Rev. 2014, 114, 11636–11682.
- Das, S.K.; Mandal, S.S.; Bhattacharyya, A.J. Ionic conductivity, mechanical strength and Li-ion battery performance of mono-functional and bi-functional (“Janus”) “soggy sand” electrolytes. Energy Environ. Sci. 2011, 4, 1391–1399.
- Cao, J.; Wang, L.; He, X.; Fang, M.; Gao, J.; Li, J.; Deng, L.; Chen, H.; Tian, G.; Wang, J. In situ prepared nano-crystalline TiO2–poly(methyl methacrylate) hybrid enhanced composite polymer electrolyte for Li-ion batteries. J. Mater. Chem. A 2013, 1, 5955–5961.
- Croce, F.; Appetecchi, G.B.; Persi, L.; Scrosati, B. Nanocomposite polymer electrolytes for lithium batteries. Nature 1998, 394, 456–458.
- Yang, J.; Zhang, H.; Zhou, Q.; Qu, DONG, T.; Zhang, M.; Tang, B.; Zhang, J.; Cui,, G. Safty -Enhanced Polymer Electrolytes for Sodium Batteries: Resent Progress and Perspectives.. ACS Appl. Mater. Interfaces. 2019, 11, 17109-17127.




