Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Carla Scorza | -- | 2156 | 2024-03-05 12:15:14 | | | |
| 2 | Jessie Wu | -1 word(s) | 2155 | 2024-03-06 02:40:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Scorza, C.A.; Goncalves, V.; Finsterer, J.; Scorza, F.; Fonseca, F. Role of Propolis in Modifying Aging Hallmarks. Encyclopedia. Available online: https://encyclopedia.pub/entry/55873 (accessed on 08 February 2026).
Scorza CA, Goncalves V, Finsterer J, Scorza F, Fonseca F. Role of Propolis in Modifying Aging Hallmarks. Encyclopedia. Available at: https://encyclopedia.pub/entry/55873. Accessed February 08, 2026.
Scorza, Carla Alessandra, Valeria Goncalves, Josef Finsterer, Fúlvio Scorza, Fernando Fonseca. "Role of Propolis in Modifying Aging Hallmarks" Encyclopedia, https://encyclopedia.pub/entry/55873 (accessed February 08, 2026).
Scorza, C.A., Goncalves, V., Finsterer, J., Scorza, F., & Fonseca, F. (2024, March 05). Role of Propolis in Modifying Aging Hallmarks. In Encyclopedia. https://encyclopedia.pub/entry/55873
Scorza, Carla Alessandra, et al. "Role of Propolis in Modifying Aging Hallmarks." Encyclopedia. Web. 05 March, 2024.
Copy Citation
Aging populations worldwide are placing age-related diseases at the forefront of the research agenda. The therapeutic potential of natural substances, especially propolis and its components, has led to these products being promising agents for alleviating several cellular and molecular-level changes associated with age-related diseases.
aging
age-related diseases
propolis
1. Propolis
Bees produce propolis, a resinous substance composed of plant elements, and apply it in stratified layers within hives to seal cracks, protect against microorganisms, regulate temperature, and encapsulate deceased intruders to prevent contamination of the colonies [1][2]. A propolis-enriched environment in bee colonies has been observed to impact bacterial populations and contribute to the stability of the bee microbiota [3]. Produced by several cataloged species, the composition of propolis varies according to geography, bee species, and local flora. Despite the potential variation among its more than 800 compounds, the samples show similarities [4]. Moreover, propolis is rich in vital vitamins, including A, D, and those from the B complex, as well as essential minerals such as calcium, magnesium, potassium, manganese, zinc, and iron. Additionally, it comprises specific fatty acids, enzymes originating from bee saliva, polysaccharides, and sugars that are frequently identified in propolis [5][6]. In the realm of organic constituents, noteworthy elements include terpenes and steroids, as well as sugars and amino acids, along with various flavonoids. Notable among the flavonoids are caffeic, cinnamic, p-coumaric, and chicoric acids, as well as quercetin, pinocembrin, baicalin, galangin, and chrysin, owing to their significant antiglycation [7][8][9], cardioprotective [10][11][12][13][14], neuroprotective [15][16][17][18][19][20], cytostatic [21][22][23], radioprotective [24][25][26][27], antimicrobial [28][29][30][31][32][33], anticancer [34][35][36][37][38][39][40], antioxidant [41][42][43][44][45][46], immunomodulatory [47][48][49][50][51][52][53], antidiabetic [54][55][56][57][58][59], and anti-inflammatory [60][61][62][63][64][65] activities and influence on gut health [50][55][66][67][68] and lipid metabolism [69][70][71][72][73][74] (Figure 1). Nevertheless, the precise mechanisms through which propolis’s bioactive constituents operate remain incompletely understood. Studies tie their effects to the mitigation of mitochondrial dysfunction, oxidative stress, inflammation, and the scavenging of free radicals. Under normal physiological circumstances, reactive oxygen species serve as cellular signaling entities involved in processes such as cell growth, adhesion, differentiation, senescence, and apoptosis. However, heightened production of oxidants is indicative of progressing inflammation [75]. Ongoing oxidative stress and inflammation are common denominators in various pathologies, including cancer, cardiovascular diseases, diabetes, neurological disorders, psychiatric illnesses, kidney diseases, lung diseases, and aging [76][77]. Elevated levels of oxidative stress, quantifiable through biomarkers, have been observed in elderly individuals or those with unhealthy lifestyles, characterized by the consumption of unwholesome diets, tobacco use, alcohol consumption, lack of physical exercise, and genetic predisposition [76][77]. The concerted action of antioxidants in ameliorating the deleterious effects of oxidative stress is achieved through the activities of antioxidant enzymes and vitamins C and E, as well as flavonoids, such as those found in propolis [78][79]. Numerous studies substantiate the potential roles and effectiveness of propolis and its compounds in counteracting the detrimental effects associated with various acute and chronic diseases [80][81][82][83][84][85][86]. The therapeutic abilities of propolis and its active components in pharmacological applications and medicine have been affirmed. Ongoing research is also rooted in a more in-depth understanding of its biological activities.
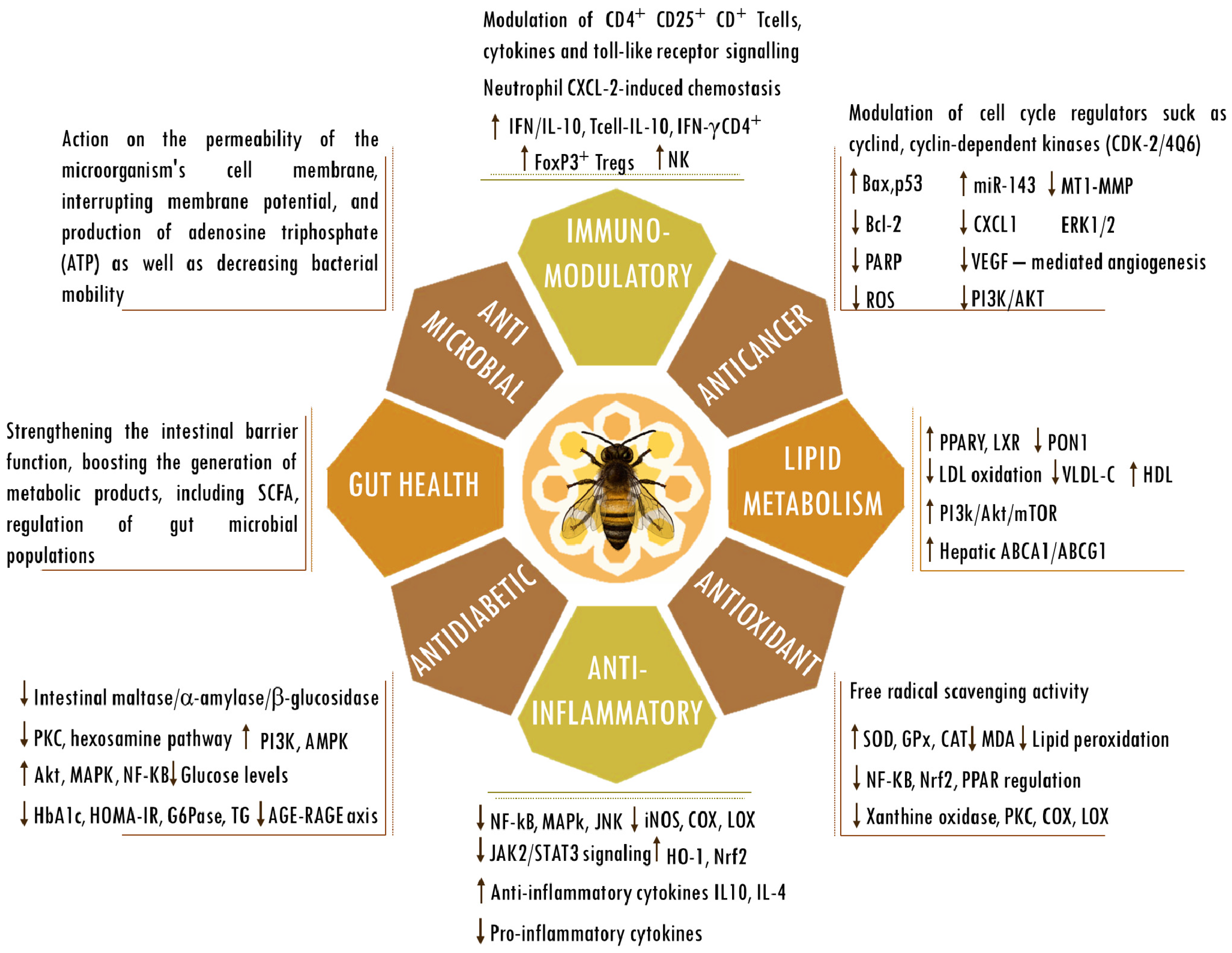
Figure 1. Biological activities of propolis. Abbreviations: ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette (ABC) subfamily G member 1; AGEs, advanced glycation end products; Bax, bcl-2-like protein 4; Bcl-2, B cell lymphoma 2; CAT, catalase; CD, cluster of differentiation; COX; cyclooxygenase; CXCL-2, chemokine (C-X-C motif) ligand 2; ERK, extracellular signal-regulated kinase; FOXP3, forkhead box P3; G6Pase, glucose-6-phosphatase; GPx, glutathione peroxidase; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; HO-1, heme oxygenase-1; HOMA-IR, homeostasis model assessment-estimated insulin resistance; iNOS, inducible nitrogen oxide synthase; IFN, interferon; IL, interleukin; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; JNK, Jun kinase; LDL, low-density lipoprotein; LOX; lipoxygenase; LXRs, liver X receptors; MDA, malondialdehyde; mTOR, mammalian target of rapamycin; MT1-MMP, membrane type 1-matrix metalloproteinase; miR143, microRNA 143; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer cells; Nrf2, nuclear factor erythroid 2-related factor 2; PPARγ, peroxisome proliferator-activated receptor gamma; PARP, poly (ADP-ribose) polymerase; p53, tumor protein p53; PI3K/AKT, phosphatidylinositol 3-kinase/ protein kinase B; PKC, protein kinase C; PON1, paraoxonase-1; RAGE, receptor for AGEs; regulatory T cells (Tregs); ROS, reactive oxygen species; SCFAs, short-chain fatty acids; SOD, superoxide dismutase; TG, triglyceride; VEGF, vascular endothelial growth factor; VLDL-c, very-low-density lipoprotein cholesterol. The up and down arrows indicate an increase or reduction in the mentioned components.
2. Genomic Stability
A stable genome is crucial for cells to reliably pass on genetic information. Accumulating genome damage and somatic mutations that induce genome instability stand out as the primary mechanisms driving aging [87]. The aging process is associated with an observed escalation in the frequency of damages to DNA (i.e., single- and double-strand breaks, crosslinks, modified bases, oxidative-induced, and depurination or depyrimidination of sugars) and mutations (i.e., insertions, deletions, or substitutions), which contribute to age-related genomic instability [88]. Cell cycle stress, alterations in gene expression, and modifications in gene regulation are outcomes of genomic instability. A broad spectrum of alterations occurs at the molecular level, including the overexpression of proinflammatory genes and inflammatory adipocytokines, impaired metabolism of glycans, glucose, and fatty acids, increases in collagen crosslinking, abnormal insulin-IGF1, mitochondrial dysfunction, disturbance of germline genetic heterogeneity, dysbiosis, and dysregulation of signaling pathways such as FoxO, heat shock proteins, NF-Κb, and mTOR signaling [89][90]. Actually, genomic instability is functionally intertwined with every hallmark of aging [91]. In the end, this might account for cellular degeneration and functional decline that occur with age. The ultimate results of genomic instability include aging and the manifestation of age-related diseases.
Genomic instability is pivotal in both the initiation and progression of cancer [92]. For instance, a visible manifestation of aging-related DNA damage is sun-damaged skin. The formation of ultraviolet light (UV)-induced DNA mutations is a crucial contributing factor to the elevated risk of cancer development associated with age [93]. Studies have shown that apigenin, an active component present in propolis, plays a crucial role in safeguarding skin keratinocytes from ultraviolet B-induced DNA damage and oxidative stress [94]. This protective effect is achieved through the activation of nucleotide excision repair genes, facilitation of cyclobutane ring repair, inhibition of ROS production, and downregulation of NF-κB and MAPK signaling pathways. The protective properties of propolis extracts are evident in human experimental in vitro skin models, where they effectively inhibit UV-induced photodamage by mitigating the occurrence of DNA strand breaks and enhancing cell viability [95]. For instance, propolis is acknowledged as a powerful inhibitor of tyrosinase, a pivotal enzyme in the melanogenesis pathway [96]. Given that UV irradiation can trigger oxidative stress leading to DNA damage and pigmentation disorders, Stavropoulou et al. examined various samples of Greek propolis. Their evaluation involved assessing free radical scavenging, anticollagenase, and antityrosinase activities to determine the antioxidant and anti-aging attributes of propolis. The research results underscored that samples featuring higher levels of flavonoids showcased superior antioxidant activity and noteworthy collagenase inhibition. In contrast, samples rich in terpenoids showed heightened antityrosinase activity [97]. Compelling evidence supports the roles of propolis and its polyphenolic components in combating cancer by countering events such as elevated DNA damage, mutations, strand breaks, chromosomal instability, deregulated DNA repair machinery, impaired cell cycle checkpoints, and dysregulated homologous recombination [98]. Recent research indicates a notable escalation in age-related hepatocellular carcinoma among older individuals [99]. Cigarette smoke-derived carcinogens such as 4-aminobiphenyl (ABP) contribute to the development of hepatocellular carcinoma in humans. The enzyme cytochrome P450 family 2 subfamily E member 1 (CYP2E1), present across mammalian species, can trigger procarcinogens and convert certain specific drugs into cytotoxic products [100]. According to research findings, propolis treatment can inhibit the conversion of the tobacco carcinogen 4-aminobiphenyl (4-ABP) into its metabolite, N-hydroxy-ABP, by inhibiting CYP2E1 expression [101]. This results in reduced production of reactive oxygen species (ROS) and genotoxicity, serving as a protective measure against cancer development. The induction of mutation, cell death, malformation, and cancer is a well-known consequence of ionizing radiation on both somatic and germ cells. Flavonoids are important components of propolis that have neuroprotective effects against brain damage caused by ionizing radiation, attributed in part to their ability to scavenge free radicals and stabilize the DNA double-helix structure [102]. A primary mechanism widely documented to counteract toxic molecules is the removal of reactive oxygen species (ROS) by antioxidants, preventing their interaction with other molecules, including DNA [93]. Studies have confirmed the powerful antioxidant capacity of propolis and its components. The SH-SY5Y cell line is frequently employed as an in vitro experimental model for investigating Parkinson’s disease. A study reported that pretreatment of SH-SY5Y cells with Brazilian green propolis decreases H2O2-generated ROS derived from mitochondria along with a reduction in the signal intensity of 8-oxo-2′-deoxyguanosine (8-oxo-dG lesion is highly mutagenic) [103].
The issue of fertility preservation has gained significant attention in the context of cancer treatment. In a mouse study, in addition to restoring testicular testosterone levels, Indian propolis also alleviated testicular toxicity caused by mitomycin-C by decreasing DNA damage and enhancing antioxidant activity [104]. In alignment with this, a study examining the genotoxicity, cytotoxicity, and clonogenic death of Chinese hamster ovary cells exposed to gamma radiation indicated the potential utility of propolis in mitigating the adverse effects caused by ionizing radiation [105]. It has been observed that apigenin becomes integrated into the nuclear matrix of prostate epithelial cells. Its binding to nucleic acid bases within the matrix is believed to underlie its antioxidant and chemopreventive activities [106].
Iron’s impact on the aging process is frequently underestimated. Despite its importance for living organisms, its reactivity poses a potential risk [107]. With aging, there is a buildup of iron that correlates with the onset of many age-related diseases. Through its strong iron-binding ability and high lipophilicity, caffeic acid phenethyl ester (CAPE), a constituent of propolis, offers protection against cellular DNA damage caused by iron [108]. Studies have demonstrated a correlation between the antioxidant effects of Portuguese propolis extracts and their ability to prevent DNA damage induced by Fe2+ [109].
Diabetes emerges as a key health issue affecting the elderly population. The literature findings suggest that the use of propolis and epigallocatechin gallate results in a significant increase in the survival rate of diabetic mice [110]. Moreover, the administration of this dietary supplementation yields a notable reduction in lipid peroxidation levels in the kidney, liver, and brain tissue, along with reduced DNA damage in the peripheral lymphocytes of diabetic animals [110].
3. Telomere Length
Unique genomic segments called telomeres reside at the extremities of linear chromosomes [111]. Their primary function is to ensure chromosome integrity and genome stability, offering protection against harm and deterioration [112]. Without a telomere maintenance mechanism, telomeres in adult somatic cells undergo a reduction in length after each round of mitotic cell division, ultimately resulting in cellular senescence and aging [113]. The length of telomeres, a complex hereditary characteristic, imposes a significant constraint on telomere function and is intricately tied to the aging process and age-related diseases [114]. Lifestyle stress and environmental factors, such as diet, can influence telomere length [115]. The results of research involving human subjects showed that prolonged and frequent intake of bee products, including propolis, is linked to telomere length [116]. In Nasir et al.’s (2015) study, which involved comparing beekeepers consuming bee products with non-beekeeper control subjects abstaining from bee products and beekeeping-related activities, it was observed that annual bee product consumption correlated with an average increase of 0.258 kbp in telomere length [116]. The results also revealed that a greater daily frequency of bee product intake was associated with an average increment of 2.66 kbp in telomere length [116]. It has been suggested that telomere length might benefit positively from an antioxidant-rich diet, as telomeres are known to be prone to shortening due to ROS-induced damage, as well as being particularly vulnerable to the prevalent lesion 8-oxo-guanine and its accumulation [117].
Quercetin is a major flavonoid compound found in propolis that has gained significant recognition for its antioxidant and anti-inflammatory properties, along with its potential anticancer and antitumor effects [118]. For instance, targeting human telomeric G-quadruplex DNA represents one of the mechanisms through which this propolis component demonstrates its anticancer activity [119]. These findings propose quercetin as a strong contender for telomere targeting and as a potent anticancer agent. Additionally, quercetin acts as a suppressor of telomerase, an enzyme responsible for the maintenance of telomere integrity and length [120][121][122]. Telomerase overexpression, a characteristic feature in almost all human cancers, plays a key role in sustaining telomere length and contributes to abnormal cell proliferation and immortalization [123]. Caffeic acid phenethyl ester (CAPE), another potent bioactive compound found in propolis, also exhibits antitumoral and antioxidant properties, as well as cytotoxic and apoptotic effects. The induction of apoptosis by CAPE has been linked to its ability to induce the activity of the catalytic subunit of telomerase, known as human telomerase reverse transcriptase [124]. In parallel, by specifically targeting leukemia cells derived from leukemia patients, Manisa propolis effectively reduces the expression of human telomerase reverse transcriptase, with chrysin identified as a key constituent responsible for this effect [125][126]. Telomerase activation in cancer has been attributed to several mechanisms, including the involvement of oncogenes like Wnt, which serve as transcriptional regulators of telomerase [123]. The ability of Aydın Turkish propolis to modulate the expression of microRNAs in glioblastoma and brain cancer stem cells underscores its anticancer effect, with the WNT and NOTCH pathways being implicated [127].
References
- Weis, W.A.; Ripari, N.; Conte, F.L.; Honorio, M.S.; Sartori, A.A.; Matucci, R.H.; Sforcin, J.M. An Overview about Apitherapy and Its Clinical Applications. Phytomed. Plus 2022, 2, 100239.
- Bobiş, O. Plants: Sources of Diversity in Propolis Properties. Plants 2022, 11, 2298.
- Saelao, P.; Borba, R.S.; Ricigliano, V.; Spivak, M.; Simone-Finstrom, M. Honeybee Microbiome Is Stabilized in the Presence of Propolis. Biol. Lett. 2020, 16, 20200003.
- Kasote, D.; Bankova, V.; Viljoen, A.M. Propolis: Chemical Diversity and Challenges in Quality Control. Proc. Phytochem. Soc. Eur. 2022, 21, 1887–1911.
- Khalil, M.L. Biological Activity of Bee Propolis in Health and Disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31.
- Rivera-Yañez, N.; Rivera-Yañez, C.R.; Pozo-Molina, G.; Méndez-Catalá, C.F.; Méndez-Cruz, A.R.; Nieto-Yañez, O. Biomedical Properties of Propolis on Diverse Chronic Diseases and Its Potential Applications and Health Benefits. Nutrients 2020, 13, 78.
- Xavier, J.A.; Valentim, I.B.; Camatari, F.O.S.; de Almeida, A.M.M.; Goulart, H.F.; Ferro, J.N.S.; Barreto, E.O.; Cavalcanti, B.C.; Bottoli, C.B.G.; Goulart, M.O.F. Polyphenol profile by UHPLC-MS/MS, anti-glycation, antioxidant and cytotoxic activities of several samples of propolis from the northeastern semi-arid region of Brazil. Pharm. Biol. 2017, 55, 1884–1893.
- Kazemi, F.; Divsalar, A.; Saboury, A.A.; Seyedarabi, A. Propolis nanoparticles prevent structural changes in human hemoglobin during glycation and fructation. Colloids Surf. B 2019, 177, 188–195.
- Egawa, T.; Ohno, Y.; Yokoyama, S.; Yokokawa, T.; Tsuda, S.; Goto, K.; Hayashi, T. The Protective Effect of Brazilian Propolis against Glycation Stress in Mouse Skeletal Muscle. Foods 2019, 8, 439.
- Goncalves, V.C.; Pinheiro, D.J.L.L.; de la Rosa, T.; de Almeida, A.G.; Scorza, F.A.; Scorza, C.A. Propolis as A Potential Disease-Modifying Strategy in Parkinson’s Disease: Cardioprotective and Neuroprotective Effects in the 6-OHDA Rat Model. Nutrients 2020, 12, 1551.
- Barary, M.; Hosseinzadeh, R.; Kazemi, S.; Liang, J.J.; Mansoori, R.; Sio, T.T.; Hosseini, M.; Moghadamnia, A.A. The effect of propolis on 5-fluorouracil-induced cardiac toxicity in rats. Sci. Rep. 2022, 12, 8661.
- Wang, Q.; Chen, R.; Tang, Q.; Chen, J.Y.; Wang, Y.; Sui, D.J.; Liu, A.D. Flavonoid Extract from Propolis Provides Cardioprotection following Myocardial Infarction by Activating PPAR-γ. Evid.-Based Complement. Altern. Med. 2022, 2022, 1333545.
- Goncalves, V.C.; Silva da Fonsêca, V.; de Paula Faria, D.; Izidoro, M.A.; Berretta, A.A.; de Almeida, A.G.; Fonseca, F.L.A.; Scorza, F.A.; Scorza, C.A. Propolis induces cardiac metabolism changes in 6-hydroxydopamine animal model: A dietary intervention as a potential cardioprotective approach in Parkinson’s disease. Front. Pharmacol. 2022, 13, 1013703.
- Maddahi, M.; Nattagh-Eshtivani, E.; Jokar, M.; Barati, M.; Tabesh, H.; Safarian, M.; Khosravi, M. The effect of propolis supplementation on cardiovascular risk factors in women with rheumatoid arthritis: A double-blind, placebo-controlled randomized clinical trial. Phytother. Res. 2023, 37, 5424–5434.
- Wang, W.; Zheng, L.; Xu, L.; Tu, J.; Gu, X. Pinocembrin mitigates depressive-like behaviors induced by chronic unpredictable mild stress through ameliorating neuroinflammation and apoptosis. Mol. Med. 2020, 26, 53.
- Palaz, M.N.; Akcay, E. The Impact of Propolis Factor Caffeic Acid Phenethyl-Ester on the Cerebral Vasospasm and Early Brain Damage in the Experimentally Induced Subarachnoid Hemorrhage on Rats. World Neurosurg. 2020, 138, e736–e742.
- Ali, A.M.; Kunugi, H. Apitherapy for Parkinson’s Disease: A Focus on the Effects of Propolis and Royal Jelly. Oxidative Med. Cell. Longev. 2020, 2020, 1727142.
- Köseoğlu Toksoy, C.; Sarıtaş, Z.K.; Türk Börü, Ü.; Zeytin Demiral, G.; Görücü, F.; Bülbül, A.; Demirel, H.H.; Koç, Y. Investigation of the protective effect of anzer propolis in cerebral ischemia-reperfusion injury. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 8004–8012.
- Ito, T.; Degawa, T.; Okumura, N. Brazilian green propolis prevents Alzheimer’s disease-like cognitive impairment induced by amyloid beta in mice. BMC Complement. Med. Ther. 2023, 23, 416.
- Mamashli, F.; Meratan, A.A.; Ghasemi, A.; Obeidi, N.; Salmani, B.; Atarod, D.; Pirhaghi, M.; Moosavi-Movahedi, F.; Mohammad-Zaheri, M.; Shahsavani, M.B.; et al. Neuroprotective Effect of Propolis Polyphenol-Based Nanosheets in Cellular and Animal Models of Rotenone-Induced Parkinson’s Disease. ACS Chem. Neurosci. 2023, 14, 851–863.
- Pratsinis, H.; Kletsas, D.; Melliou, E.; Chinou, I. Antiproliferative activity of Greek propolis. J. Med. Food 2010, 13, 286–290.
- Rajendran, P.; Rengarajan, T.; Nandakumar, N.; Palaniswami, R.; Nishigaki, Y.; Nishigaki, I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014, 86, 103–112.
- Wang, C.C.; Wang, Y.X.; Yu, N.Q.; Hu, D.; Wang, X.Y.; Chen, X.G.; Liao, Y.W.; Yao, J.; Wang, H.; He, L.; et al. Brazilian Green Propolis Extract Synergizes with Protoporphyrin IX-mediated Photodynamic Therapy via Enhancement of Intracellular Accumulation of Protoporphyrin IX and Attenuation of NF-κB and COX-2. Molecules 2017, 22, 732.
- Benković, V.; Knezević, A.H.; Dikić, D.; Lisicić, D.; Orsolić, N.; Basić, I.; Kopjar, N. Radioprotective effects of quercetin and ethanolic extract of propolis in gamma-irradiated mice. Arh. Hig. Rada Toksikol. 2009, 60, 129–138.
- Yalcin, C.O.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Bahat, Z.; Misir, S.; Deger, O. Evaluation of the radioprotective effect of Turkish propolis on foreskin fibroblast cells. J. Cancer Res. Ther. 2016, 12, 990–994.
- Avci, G.G.; Erdim, I.; Ozmen, Z.C.; Gevrek, F.; Colak, S.; Demirsoy, M.S.; Bozkurt, H. The effect of systemic application of propolis on tongue damage and oral mucositis in rats exposed to radiation. Eur. Arch. Otorhinolaryngol. 2022, 279, 1043–1052.
- Ibáñez, B.; Melero, A.; Montoro, A.; San Onofre, N.; Soriano, J.M. Radioprotective Effects from Propolis: A Review. Molecules 2023, 28, 5842.
- Aytekin, A.A.; Tanrıverdi, S.T.; Köse, F.A.; Kart, D.; Eroğlu, İ.; Özer, Ö. Propolis loaded liposomes: Evaluation of antimicrobial and antioxidant activities. J. Liposome Res. 2020, 30, 107–116.
- Navarro-Pérez, M.L.; Vadillo-Rodríguez, V.; Fernández-Babiano, I.; Pérez-Giraldo, C.; Fernández-Calderón, M.C. Antimicrobial activity of a novel Spanish propolis against planktonic and sessile oral Streptococcus spp. Sci. Rep. 2021, 11, 23860.
- Sampaio, G.A.M.; Lacerda-Santos, R.; Cavalcanti, Y.W.; Vieira, G.H.A.; Nonaka, C.F.W.; Alves, P.M. Antimicrobial properties, mechanics, and fluoride release of ionomeric cements modified by red propolis. Angle Orthod. 2021, 91, 522–527.
- Silva, T.S.; Silva, J.M.B.; Braun, G.H.; Mejia, J.A.A.; Capatinta, G.V.C.; Santos, M.F.C.; Tanimoto, M.H.; Bastos, J.K.; Parreira, R.L.T.; Orenha, R.P.; et al. Green and Red Brazilian Propolis: Antimicrobial Potential and Anti-Virulence against ATCC and Clinically Isolated Multidrug-Resistant Bacteria. Chem. Biodivers. 2021, 18, e2100307.
- Assis, M.A.S.; Ramos, L.P.; Hasna, A.A.; de Queiroz, T.S.; Pereira, T.C.; Lima, P.M.N.; Berretta, A.A.; Marcucci, M.C.; Carvalho, C.A.T.; Oliveira, L.D. Antimicrobial and Antibiofilm Effect of Brazilian Green Propolis Aqueous Extract against Dental Anaerobic Bacteria. Molecules 2022, 27, 8128.
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Paluch, E.; Sakipov, A.; Zhumashova, G.; Ibadullayeva, G.; Sakipova, Z.; Korona-Glowniak, I. Phytochemical Profile and Antimicrobial Potential of Propolis Samples from Kazakhstan. Molecules 2023, 28, 2984.
- Seyhan, M.F.; Yılmaz, E.; Timirci-Kahraman, Ö.; Saygılı, N.; Kısakesen, H.İ.; Gazioğlu, S.; Gören, A.C.; Eronat, A.P.; Ceviz, A.B.; Öztürk, T.; et al. Different propolis samples, phenolic content, and breast cancer cell lines: Variable cytotoxicity ranging from ineffective to potent. IUBMB Life 2019, 71, 619–631.
- Frión-Herrera, Y.; Gabbia, D.; Scaffidi, M.; Zagni, L.; Cuesta-Rubio, O.; De Martin, S.; Carrara, M. Cuban brown propolis interferes in the crosstalk between colorectal cancer cells and M2 macrophages. Nutrients 2020, 12, 2040.
- Misir, S.; Aliyazicioglu, Y.; Demir, S.; Turan, I.; Hepokur, C. Effect of Turkish Propolis on miRNA Expression, Cell Cycle, and Apoptosis in Human Breast Cancer (MCF-7) Cells. Nutr. Cancer 2020, 72, 133–145.
- Salem, M.M.; Donia, T.; Abu-Khudir, R.; Ramadan, H.; Ali, E.M.M.; Mohamed, T.M. Propolis Potentiates Methotrexate Anticancer Mechanism and Reduces its Toxic Effects. Nutr. Cancer 2020, 72, 460–480.
- Alanazi, S.; Alenzi, N.; Alenazi, F.; Tabassum, H.; Watson, D. Chemical characterization of Saudi propolis and its antiparasitic and anticancer properties. Sci. Rep. 2021, 11, 5390.
- AlDreini, S.; Fatfat, Z.; Abou Ibrahim, N.; Fatfat, M.; Gali-Muhtasib, H.; Khalife, H. Thymoquinone enhances the antioxidant and anticancer activity of Lebanese propolis. World J. Clin. Oncol. 2023, 14, 203–214.
- Shen, M.H.; Liu, C.Y.; Chang, K.W.; Lai, C.L.; Chang, S.C.; Huang, C.J. Propolis Has an Anticancer Effect on Early Stage Colorectal Cancer by Affecting Epithelial Differentiation and Gut Immunity in the Tumor Microenvironment. Nutrients 2023, 15, 4494.
- Chi, Y.; Luo, L.; Cui, M.; Hao, Y.; Liu, T.; Huang, X.; Guo, X. Chemical Composition and Antioxidant Activity of Essential Oil of Chinese Propolis. Chem. Biodivers. 2020, 17, e1900489.
- Alaribe, C.S.; Esposito, T.; Sansone, F.; Sunday, A.; Pagano, I.; Piccinelli, A.L.; Celano, R.; Cuesta Rubio, O.; Coker, H.A.; Nabavi, S.M.; et al. Nigerian propolis: Chemical composition, antioxidant activity and α-amylase and α-glucosidase inhibition. Nat. Prod. Res. 2021, 35, 3095–3099.
- Boufadi, M.Y.; Soubhye, J.; Van Antwerpen, P. Anti-inflammatory, antioxidant effects, and bioaccessibility of Tigzirt propolis. J. Food Biochem. 2021, 45, e13663.
- Salehi, A.; Hosseini, S.M.; Kazemi, S. Antioxidant and Anticarcinogenic Potentials of Propolis for Dimethylhydrazine-Induced Colorectal Cancer in Wistar Rats. Biomed Res. Int. 2022, 2022, 8497562.
- Popova, M.; Trusheva, B.; Chimshirova, R.; Antonova, D.; Gechovska, K.; Thanh, L.N.; Lien, N.T.P.; Phuong, D.T.L.; Bankova, V. Chemical Profile and Antioxidant Capacity of Propolis from Tetragonula, Lepidotrigona, Lisotrigona and Homotrigona Stingless Bee Species in Vietnam. Molecules 2022, 27, 7834.
- Arung, E.T.; Syafrizal; Kusuma, I.W.; Paramita, S.; Amen, Y.; Kim, Y.U.; Naibaho, N.M.; Ramadhan, R.; Ariyanta, H.A.; Fatriasari, W.; et al. Antioxidant, anti-inflammatory and anti-acne activities of stingless bee (Tetragonula biroi) propolis. Fitoterapia 2023, 164, 105375.
- Bachiega, T.F.; Orsatti, C.L.; Pagliarone, A.C.; Sforcin, J.M. The effects of propolis and its isolated compounds on cytokine production by murine macrophages. Phytother. Res. 2012, 26, 1308–1313.
- Sforcin, J.M. Biological properties and therapeutic applications of propolis. Phytother. Res. 2016, 30, 894–905.
- Santos, L.A.; Rosalen, P.L.; Dias, N.A.; Gomes, B.J.N.; Blosfeld-Lopes, L.; Ikegaki, M.; de Alencar, S.M.; Burger, E. Brazilian Red Propolis shows antifungal and immunomodulatory activities against Paracoccidioides brasiliensis. J. Ethnopharmacol. 2021, 277, 114181.
- Okamura, T.; Hamaguchi, M.; Bamba, R.; Nakajima, H.; Yoshimura, Y.; Kimura, T.; Hashimoto, Y.; Majima, S.; Senmaru, T.; Ushigome, E.; et al. Brazilian Green Propolis Improves Gut Microbiota Dysbiosis and Protects against Sarcopenic Obesity. J. Cachexia Sarcopenia Muscle 2022, 13, 3028–3047.
- Al-Kahtani, S.N.; Alaqil, A.A.; Abbas, A.O. Modulation of Antioxidant Defense, Immune Response, and Growth Performance by Inclusion of Propolis and Bee Pollen into Broiler Diets. Animals 2022, 12, 1658.
- Rebouças-Silva, J.; Amorim, N.A.; Jesus-Santos, F.H.; de Lima, J.A.; Lima, J.B.; Berretta, A.A.; Borges, V.M. Leishmanicidal and immunomodulatory properties of Brazilian green propolis extract (EPP-AF®) and a gel formulation in a pre-clinical model. Front. Pharmacol. 2023, 14, 1013376.
- Abdel-Maksoud, E.M.; Daha, A.A.E.F.; Taha, N.M.; Lebda, M.A.; Sadek, K.M.; Alshahrani, M.Y.; Ahmed, A.E.; Shukry, M.; Fadl, S.E.; Elfeky, M. Effects of ginger extract and/or propolis extract on immune system parameters of vaccinated broilers. Poult. Sci. 2023, 102, 102903.
- Usman, U.Z.; Bakar, A.B.A.; Mohamed, M. Propolis improves pregnancy outcomes and placental oxidative stress status in streptozotocin-induced diabetic rats. BMC Complement. Altern. Med. 2018, 18, 324.
- Xue, M.; Liu, Y.; Xu, H.; Zhou, Z.; Ma, Y.; Sun, T.; Liu, M.; Zhang, H.; Liang, H. Propolis modulates the gut microbiota and improves the intestinal mucosal barrier function in diabetic rats. Biomed. Pharmacother. 2019, 118, 109393.
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Teixeira, J.A.; Lyoussi, B. Effect of antioxidant-rich propolis and bee pollen extracts against D-glucose induced type 2 diabetes in rats. Food Res. Int. 2020, 138 Pt B, 109802.
- Taleb, R.A.; Djebli, N.; Chenini, H.; Sahin, H.; Kolayli, S. In vivo and in vitro anti-diabetic activity of ethanolic propolis extract. J. Food Biochem. 2020, 44, e13267.
- Salrian, A.A.; Behzadi, A.; Oloumi, M.M.; Farajli Abbasi, M.; Delshad, S.; Moghadaszadeh, M. Amplification of Wound Healing by Propolis and Honey Ointment in Healthy and Diabetic Rat Models; Histopathological and Morphometric Findings. Arch. Razi Inst. 2022, 77, 1673–1681.
- Ochoa-Morales, P.D.; González-Ortiz, M.; Martínez-Abundis, E.; Pérez-Rubio, K.G.; Patiño-Laguna, A.D.J. Anti-hyperglycemic effects of propolis or metformin in type 2 Diabetes Mellitus.International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. Int. J. Vitam. Nutr. Res. 2023, 93, 498–506.
- Batista, C.M.; Alves, A.V.F.; Queiroz, L.A.; Lima, B.S.; Filho, R.N.P.; Araújo, A.A.S.; de Albuquerque Júnior, R.L.C.; Cardoso, J.C. The photoprotective and anti-inflammatory activity of red propolis extract in rats. J. Photochem. Photobiol. B Biol. 2018, 180, 198–207.
- Song, M.Y.; Lee, D.Y.; Kim, E.H. Anti-inflammatory and anti-oxidative effect of Korean propolis on Helicobacter pylori-induced gastric damage in vitro. J. Microbiol. 2020, 58, 878–885.
- Sokeng, S.D.; Talla, E.; Sakava, P.; Fokam Tagne, M.A.; Henoumont, C.; Sophie, L.; Mbafor, J.T.; Tchuenguem Fohouo, F.N. Anti-Inflammatory and Analgesic Effect of Arachic Acid Ethyl Ester Isolated from Propolis. Biomed Res. Int. 2020, 2020, 8797284.
- Inui, S.; Hosoya, T.; Yoshizumi, K.; Sato, H.; Kumazawa, S. Phytochemical and anti-inflammatory properties of Senegalese propolis and isolated compounds. Fitoterapia 2021, 151, 104861.
- Mendez-Encinas, M.A.; Valencia, D.; Ortega-García, J.; Carvajal-Millan, E.; Díaz-Ríos, J.C.; Mendez-Pfeiffer, P.; Soto-Bracamontes, C.M.; Garibay-Escobar, A.; Alday, E.; Velazquez, C. Anti-Inflammatory Potential of Seasonal Sonoran Propolis Extracts and Some of Their Main Constituents. Molecules 2023, 28, 4496.
- Valverde, T.M.; Soares, B.N.G.S.; Nascimento, A.M.D.; Andrade, Â.L.; Sousa, L.R.D.; Vieira, P.M.A.; Santos, V.R.; Seibert, J.B.; Almeida, T.C.S.; Rodrigues, C.F.; et al. Anti-Inflammatory, Antimicrobial, Antioxidant and Photoprotective Investigation of Red Propolis Extract as Sunscreen Formulation in Polawax Cream. Int. J. Mol. Sci. 2023, 24, 5112.
- Batista, M.A.C.; Braga, D.C.A.; Moura, S.A.L.; Souza, G.H.B.; Santos, O.D.H.; Cardoso, L.M. Salt-dependent hypertension and inflammation: Targeting the gut-brain axis and the immune system with Brazilian green propolis. Inflammopharmacology 2020, 28, 1163–1182.
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939.
- Garzarella, E.U.; Navajas-Porras, B.; Pérez-Burillo, S.; Ullah, H.; Esposito, C.; Santarcangelo, C.; Hinojosa-Nogueira, D.; Pastoriza, S.; Zaccaria, V.; Xiao, J.; et al. Evaluating the effects of a standardized polyphenol mixture extracted from poplar-type propolis on healthy and diseased human gut microbiota. Biomed. Pharmacother. 2022, 148, 112759.
- Nakajima, M.; Arimatsu, K.; Minagawa, T.; Matsuda, Y.; Sato, K.; Takahashi, N.; Nakajima, T.; Yamazaki, K. Brazilian propolis mitigates impaired glucose and lipid metabolism in experimental periodontitis in mice. BMC Complement. Altern. Med. 2016, 16, 329.
- Mujica, V.; Orrego, R.; Pérez, J.; Romero, P.; Ovalle, P.; Zúñiga-Hernández, J.; Arredondo, M.; Leiva, E. The Role of Propolis in Oxidative Stress and Lipid Metabolism: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 4272940.
- Huang, X.; Wu, X.; Yan, S.; Lan, T. Lipid-lowering effect of propolis in mice with Triton-WR1339-induced hyperlipidemia and its mechanism for regulating lipid metabolism. J. South. Med. 2018, 38, 1020–1024.
- Zakerkish, M.; Jenabi, M.; Zaeemzadeh, N.; Hemmati, A.A.; Neisi, N. The Effect of Iranian Propolis on Glucose Metabolism, Lipid Profile, Insulin Resistance, Renal Function and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Sci. Rep. 2019, 9, 7289.
- Kong, L.; Zhang, Y.; Feng, Z.; Dong, J.; Zhang, H. Phenolic Compounds of Propolis Alleviate Lipid Metabolism Disorder. Evid.-Based Complement. Altern. Med. 2021, 2021, 7615830.
- Zhao, L.; Yao, L.; Chen, R.; He, T.; Qiu, S.; Chen, G.; Chen, H.; Qiu, S.-X. Pinostrobin from plants and propolis against human coronavirus HCoV-OC43 by modulating host AHR/CYP1A1 pathway and lipid metabolism. Antiviral Res. 2023, 212, 105570.
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The Interplay of Oxidative Stress and Inflammation: Mechanistic Insights and Therapeutic Potential of Antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914.
- Baierle, M.; Nascimento, S.N.; Moro, A.M.; Brucker, N.; Freitas, F.; Gauer, B.; Durgante, J.; Bordignon, S.; Zibetti, M.; Trentini, C.M.; et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxidative Med. Cell. Longev. 2015, 2015, 804198.
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dąbrowski, A.; Kędziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxidative Med. Cell. Longev. 2019, 2019, 5123628.
- Ahangari, Z.; Naseri, M.; Vatandoost, F. Propolis: Chemical Composition and Its Applications in Endodontics. Iran. Endod. J. 2018, 13, 285–292.
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100.
- Zulhendri, F.; Perera, C.O.; Tandean, S. Can Propolis Be a Useful Adjuvant in Brain and Neurological Disorders and Injuries? A Systematic Scoping Review of the Latest Experimental Evidence. Biomedicines 2021, 9, 1227.
- Zulhendri, F.; Ravalia, M.; Kripal, K.; Chandrasekaran, K.; Fearnley, J.; Perera, C.O. Propolis in Metabolic Syndrome and Its Associated Chronic Diseases: A Narrative Review. Antioxidants 2021, 10, 348.
- Silva, H.; Francisco, R.; Saraiva, A.; Francisco, S.; Carrascosa, C.; Raposo, A. The Cardiovascular Therapeutic Potential of Propolis—A Comprehensive Review. Biology 2021, 10, 27.
- Forma, E.; Bryś, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594.
- Pahlavani, N.; Malekahmadi, M.; Firouzi, S.; Rostami, D.; Sedaghat, A.; Moghaddam, A.B.; Ferns, G.A.; Navashenaq, J.G.; Reazvani, R.; Safarian, M.; et al. Molecular and Cellular Mechanisms of the Effects of Propolis in Inflammation, Oxidative Stress, and Glycemic Control in Chronic Diseases. Nutr. Metab. 2020, 17, 65.
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120.
- Chavda, V.P.; Chaudhari, A.Z.; Teli, D.; Balar, P.; Vora, L. Propolis and Their Active Constituents for Chronic Diseases. Biomedicines 2023, 11, 259.
- Maynard, S.; Fang, E.F.; Scheibye-Knudsen, M.; Croteau, D.L.; Bohr, V.A. DNA Damage, DNA Repair, Aging, and Neurodegeneration. Cold Spring Harb. Perspect. Med. 2015, 5, a025130.
- Laffon, B.; Bonassi, S.; Costa, S.; Valdiglesias, V. Genomic instability as a main driving factor of unsuccessful ageing: Potential for translating the use of micronuclei into clinical practice. Mutat. Res. Rev. 2021, 787, 108359.
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391.
- Rattan, S.I.S. Molecular Basis of Nutrition and Aging: Molecular and Cellular Basis of Aging; Academic Press: New York, NY, USA, 2016; pp. 3–9.
- López-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279.
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, 5–24.
- Petr, M.A.; Tulika, T.; Carmona-Marin, L.M.; Scheibye-Knudsen, M. Protecting the Aging Genome. Trends Cell Biol. 2020, 30, 117–132.
- Das, S.; Das, J.; Paul, A.; Samadder, A.; Khuda-Bukhsh, A.R. Apigenin, a bioactive flavonoid from Lycopodium clavatum, stimulates nucleotide excision repair genes to protect skin keratinocytes from ultraviolet B-induced reactive oxygen species and DNA damage. J. Acupunct. Meridian Stud. 2013, 6, 252–262.
- Karapetsas, A.; Voulgaridou, G.P.; Konialis, M.; Tsochantaridis, I.; Kynigopoulos, S.; Lambropoulou, M.; Stavropoulou, M.I.; Stathopoulou, K.; Aligiannis, N.; Bozidis, P.; et al. Propolis Extracts Inhibit UV-Induced Photodamage in Human Experimental In Vitro Skin Models. Antioxidants 2019, 8, 125.
- Widelski, J.; Gaweł-Bęben, K.; Czech, K.; Paluch, E.; Bortkiewicz, O.; Kozachok, S.; Mroczek, T.; Okińczyc, P. Extracts from European Propolises as Potent Tyrosinase Inhibitors. Molecules 2022, 28, 55.
- Stavropoulou, M.I.; Stathopoulou, K.; Cheilari, A.; Benaki, D.; Gardikis, K.; Chinou, I.; Aligiannis, N. NMR metabolic profiling of Greek propolis samples: Comparative evaluation of their phytochemical compositions and investigation of their anti-ageing and antioxidant properties. J. Pharm. Biomed. Anal. 2021, 194, 113814.
- Oršolić, N.; Jazvinšćak Jembrek, M. Molecular and Cellular Mechanisms of Propolis and Its Polyphenolic Compounds against Cancer. Int. J. Mol. Sci. 2022, 23, 10479.
- Cho, E.; Cho, H.A.; Jun, C.H.; Kim, H.J.; Cho, S.B.; Choi, S.K. A Review of Hepatocellular Carcinoma in Elderly Patients Focused on Management and Outcomes. In Vivo 2019, 33, 1411–1420.
- Harjumäki, R.; Pridgeon, C.S.; Ingelman-Sundberg, M. CYP2E1 in Alcoholic and Non-Alcoholic Liver Injury. Roles of ROS, Reactive Intermediates and Lipid Overload. Int. J. Mol. Sci. 2021, 22, 8221.
- Wahyuni, E.A.; Chen, C.Y.; Wu, H.N.; Chien, C.C.; Chen, S.C. Propolis alleviates 4-aminobiphenyl-induced oxidative DNA damage by inhibition of CYP2E1 expression in human liver cells. Environ. Toxicol. 2021, 36, 1504–1513.
- Wang, Q.; Xie, C.; Xi, S.; Qian, F.; Peng, X.; Huang, J.; Tang, F. Radioprotective Effect of Flavonoids on Ionizing Radiation-Induced Brain Damage. Molecules 2020, 25, 5719.
- Ni, J.; Wu, Z.; Meng, J.; Zhu, A.; Zhong, X.; Wu, S.; Nakanishi, H. The Neuroprotective Effects of Brazilian Green Propolis on Neurodegenerative Damage in Human Neuronal SH-SY5Y Cells. Oxidative Med. Cell. Longev. 2017, 2017, 7984327.
- Kumari, S.; Nayak, G.; Lukose, S.T.; Kalthur, S.G.; Bhat, N.; Hegde, A.R.; Mutalik, S.; Kalthur, G.; Adiga, S.K. Indian propolis ameliorates the mitomycin C-induced testicular toxicity by reducing DNA damage and elevating the antioxidant activity. Biomed. Pharmacother. 2017, 95, 252–263.
- Santos, G.S.; Tsutsumi, S.; Vieira, D.P.; Bartolini, P.; Okazaki, K. Effect of Brazilian propolis (AF-08) on genotoxicity, cytotoxicity and clonogenic death of Chinese hamster ovary (CHO-K1) cells irradiated with (60)Co gamma-radiation. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2014, 762, 17–23.
- Sharma, H.; Kanwal, R.; Bhaskaran, N.; Gupta, S. Plant flavone apigenin binds to nucleic acid bases and reduces oxidative DNA damage in prostate epithelial cells. PLoS ONE 2014, 9, e91588.
- Mangan, D. Iron: An underrated factor in aging. Aging 2021, 13, 23407–23415.
- Shao, B.; Mao, L.; Tang, M.; Yan, Z.-Y.; Shao, J.; Huang, C.-H.; Sheng, Z.-G.; Zhu, B.-Z. Caffeic Acid Phenyl Ester (CAPE) Protects against Iron-Mediated Cellular DNA Damage through Its Strong Iron-Binding Ability and High Lipophilicity. Antioxidants 2021, 10, 798.
- Barroso, L.; Puškár, M.; Ševčovičová, A.; Almeida-Aguiar, C.; Oliveira, R. Analysis of the effects of propolis extracts on DNA damage. In Študentská Vedecká Konferencia; Univerzita Komenského v Bratislave: Bratislava, Slovakia, 2016; pp. 20–25.
- Oršolić, N.; Sirovina, D.; Gajski, G.; Garaj-Vrhovac, V.; Jembrek, M.J.; Kosalec, I. Assessment of DNA damage and lipid peroxidation in diabetic mice: Effects of propolis and epigallocatechin gallate (EGCG). Mutat. Res. 2013, 757, 36–44.
- Rossiello, F.; Jurk, D.; Passos, J.F.; Fagagna, F.A. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147.
- Lee, J.; Pellegrini, M.V. Biochemistry, Telomere and Telomerase; StatPearls: Treasure Island, FL, USA, 2022; p. 35015454.
- Shay, J.W.; Wright, W.E. Telomeres and telomerase: Three decades of progress. Nat. Rev. Genet. 2019, 20, 299–309.
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558.
- Galiè, S.; Canudas, S.; Muralidharan, J.; García-Gavilán, J.; Bulló, M.; Salas-Salvadó, J. Impact of Nutrition on Telomere Health: Systematic Review of Observational Cohort Studies and Randomized Clinical Trials. Adv. Nutr. 2020, 11, 576–601.
- Nasir, N.F.; Kannan, T.P.; Sulaiman, S.A.; Shamsuddin, S.; Azlina, A.; Stangaciu, S. The relationship between telomere length and beekeeping among Malaysians. Age 2015, 37, 9797.
- Sarkar, J.; Liu, Y. The origin of oxidized guanine resolves the puzzle of oxidation-induced telomere-length alterations. Nat. Struct. Mol. Biol. 2016, 23, 1070–1071.
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-β-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134.
- Tawani, A.; Kumar, A. Structural Insight into the interaction of Flavonoids with Human Telomeric Sequence. Sci. Rep. 2015, 5, 17574.
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13.
- Parekh, N.; Garg, A.; Choudhary, R.; Gupta, M.; Kaur, G.; Ramniwas, S.; Shahwan, M.; Tuli, H.S.; Sethi, G. The Role of Natural Flavonoids as Telomerase Inhibitors in Suppressing Cancer Growth. Pharmaceuticals 2023, 16, 605.
- Bhatiya, M.; Pathak, S.; Jothimani, G.; Duttaroy, A.K.; Banerjee, A.A. Comprehensive Study on the Anti-cancer Effects of Quercetin and Its Epigenetic Modifications in Arresting Progression of Colon Cancer Cell Proliferation. Arch. Immunol. Ther. Exp. 2023, 71, 6.
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808.
- Avci, C.B.; Sahin, F.; Gunduz, C.; Selvi, N.; Aydin, H.H.; Oktem, G.; Topcuoglu, N.; Saydam, G. Protein phosphatase 2A (PP2A) has a potential role in CAPE-induced apoptosis of CCRF-CEM cells via affecting human telomerase reverse transcriptase activity. Hematology 2007, 12, 519–525.
- Gunduz, C.; Biray, C.; Kosova, B.; Yilmaz, B.; Eroglu, Z.; Sahin, F.; Omay, S.B.; Cogulu, O. Evaluation of Manisa propolis effect on leukemia cell line by telomerase activity. Leuk. Res. 2005, 29, 1343–1346.
- Cogulu, O.; Biray, C.; Gunduz, C.; Karaca, E.; Aksoylar, S.; Sorkun, K.; Salih, B.; Ozkinay, F. Effects of Manisa propolis on telomerase activity in leukemia cells obtained from the bone marrow of leukemia patients. Int. J. Food Sci. Nutr. 2009, 60, 601–605.
- Yilmaz, U.C.; Bagca, B.G.; Karaca, E.; Durmaz, A.; Durmaz, B.; Aykut, A.; Kayalar, H.; Avci, C.B.; Susluer, S.Y.; Pariltay, E.; et al. Propolis Extract Regulates microRNA Expression in Glioblastoma and Brain Cancer Stem Cells. Anticancer Agents Med. Chem. 2022, 22, 378–389.
More
Information
Subjects:
Integrative & Complementary Medicine
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
743
Revisions:
2 times
(View History)
Update Date:
06 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




