Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Integrative & Complementary Medicine
Aging populations worldwide are placing age-related diseases at the forefront of the research agenda. The therapeutic potential of natural substances, especially propolis and its components, has led to these products being promising agents for alleviating several cellular and molecular-level changes associated with age-related diseases.
- aging
- age-related diseases
- propolis
1. Propolis
Bees produce propolis, a resinous substance composed of plant elements, and apply it in stratified layers within hives to seal cracks, protect against microorganisms, regulate temperature, and encapsulate deceased intruders to prevent contamination of the colonies [31,32]. A propolis-enriched environment in bee colonies has been observed to impact bacterial populations and contribute to the stability of the bee microbiota [33]. Produced by several cataloged species, the composition of propolis varies according to geography, bee species, and local flora. Despite the potential variation among its more than 800 compounds, the samples show similarities [34]. Moreover, propolis is rich in vital vitamins, including A, D, and those from the B complex, as well as essential minerals such as calcium, magnesium, potassium, manganese, zinc, and iron. Additionally, it comprises specific fatty acids, enzymes originating from bee saliva, polysaccharides, and sugars that are frequently identified in propolis [35,36]. In the realm of organic constituents, noteworthy elements include terpenes and steroids, as well as sugars and amino acids, along with various flavonoids. Notable among the flavonoids are caffeic, cinnamic, p-coumaric, and chicoric acids, as well as quercetin, pinocembrin, baicalin, galangin, and chrysin, owing to their significant antiglycation [37,38,39], cardioprotective [40,41,42,43,44], neuroprotective [45,46,47,48,49,50], cytostatic [51,52,53], radioprotective [54,55,56,57], antimicrobial [58,59,60,61,62,63], anticancer [64,65,66,67,68,69,70], antioxidant [71,72,73,74,75,76], immunomodulatory [77,78,79,80,81,82,83], antidiabetic [84,85,86,87,88,89], and anti-inflammatory [90,91,92,93,94,95] activities and influence on gut health [80,85,96,97,98] and lipid metabolism [99,100,101,102,103,104] (Figure 1). Nevertheless, the precise mechanisms through which propolis’s bioactive constituents operate remain incompletely understood. Studies tie their effects to the mitigation of mitochondrial dysfunction, oxidative stress, inflammation, and the scavenging of free radicals. Under normal physiological circumstances, reactive oxygen species serve as cellular signaling entities involved in processes such as cell growth, adhesion, differentiation, senescence, and apoptosis. However, heightened production of oxidants is indicative of progressing inflammation [105]. Ongoing oxidative stress and inflammation are common denominators in various pathologies, including cancer, cardiovascular diseases, diabetes, neurological disorders, psychiatric illnesses, kidney diseases, lung diseases, and aging [106,107]. Elevated levels of oxidative stress, quantifiable through biomarkers, have been observed in elderly individuals or those with unhealthy lifestyles, characterized by the consumption of unwholesome diets, tobacco use, alcohol consumption, lack of physical exercise, and genetic predisposition [106,107]. The concerted action of antioxidants in ameliorating the deleterious effects of oxidative stress is achieved through the activities of antioxidant enzymes and vitamins C and E, as well as flavonoids, such as those found in propolis [108,109]. Numerous studies substantiate the potential roles and effectiveness of propolis and its compounds in counteracting the detrimental effects associated with various acute and chronic diseases [25,26,27,28,110,111,112]. The therapeutic abilities of propolis and its active components in pharmacological applications and medicine have been affirmed. Ongoing research is also rooted in a more in-depth understanding of its biological activities.
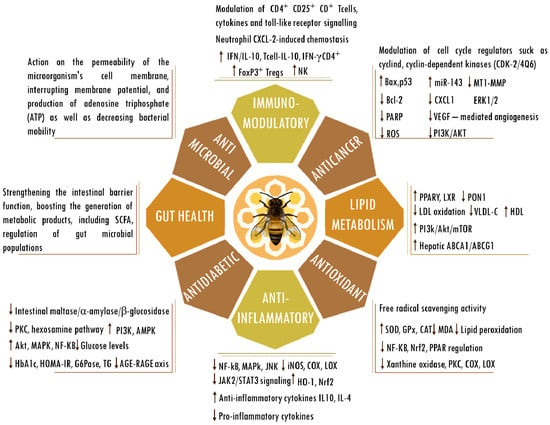
Figure 1. Biological activities of propolis. Abbreviations: ABCA1, ATP-binding cassette subfamily A member 1; ABCG1, ATP-binding cassette (ABC) subfamily G member 1; AGEs, advanced glycation end products; Bax, bcl-2-like protein 4; Bcl-2, B cell lymphoma 2; CAT, catalase; CD, cluster of differentiation; COX; cyclooxygenase; CXCL-2, chemokine (C-X-C motif) ligand 2; ERK, extracellular signal-regulated kinase; FOXP3, forkhead box P3; G6Pase, glucose-6-phosphatase; GPx, glutathione peroxidase; HbA1C, hemoglobin A1C; HDL, high-density lipoprotein; HO-1, heme oxygenase-1; HOMA-IR, homeostasis model assessment-estimated insulin resistance; iNOS, inducible nitrogen oxide synthase; IFN, interferon; IL, interleukin; JAK2/STAT3, Janus kinase 2/signal transducer and activator of transcription 3; JNK, Jun kinase; LDL, low-density lipoprotein; LOX; lipoxygenase; LXRs, liver X receptors; MDA, malondialdehyde; mTOR, mammalian target of rapamycin; MT1-MMP, membrane type 1-matrix metalloproteinase; miR143, microRNA 143; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NK, natural killer cells; Nrf2, nuclear factor erythroid 2-related factor 2; PPARγ, peroxisome proliferator-activated receptor gamma; PARP, poly (ADP-ribose) polymerase; p53, tumor protein p53; PI3K/AKT, phosphatidylinositol 3-kinase/ protein kinase B; PKC, protein kinase C; PON1, paraoxonase-1; RAGE, receptor for AGEs; regulatory T cells (Tregs); ROS, reactive oxygen species; SCFAs, short-chain fatty acids; SOD, superoxide dismutase; TG, triglyceride; VEGF, vascular endothelial growth factor; VLDL-c, very-low-density lipoprotein cholesterol. The up and down arrows indicate an increase or reduction in the mentioned components.
2. Genomic Stability
A stable genome is crucial for cells to reliably pass on genetic information. Accumulating genome damage and somatic mutations that induce genome instability stand out as the primary mechanisms driving aging [113]. The aging process is associated with an observed escalation in the frequency of damages to DNA (i.e., single- and double-strand breaks, crosslinks, modified bases, oxidative-induced, and depurination or depyrimidination of sugars) and mutations (i.e., insertions, deletions, or substitutions), which contribute to age-related genomic instability [114]. Cell cycle stress, alterations in gene expression, and modifications in gene regulation are outcomes of genomic instability. A broad spectrum of alterations occurs at the molecular level, including the overexpression of proinflammatory genes and inflammatory adipocytokines, impaired metabolism of glycans, glucose, and fatty acids, increases in collagen crosslinking, abnormal insulin-IGF1, mitochondrial dysfunction, disturbance of germline genetic heterogeneity, dysbiosis, and dysregulation of signaling pathways such as FoxO, heat shock proteins, NF-Κb, and mTOR signaling [14,115]. Actually, genomic instability is functionally intertwined with every hallmark of aging [116]. In the end, this might account for cellular degeneration and functional decline that occur with age. The ultimate results of genomic instability include aging and the manifestation of age-related diseases.
Genomic instability is pivotal in both the initiation and progression of cancer [117]. For instance, a visible manifestation of aging-related DNA damage is sun-damaged skin. The formation of ultraviolet light (UV)-induced DNA mutations is a crucial contributing factor to the elevated risk of cancer development associated with age [118]. Studies have shown that apigenin, an active component present in propolis, plays a crucial role in safeguarding skin keratinocytes from ultraviolet B-induced DNA damage and oxidative stress [119]. This protective effect is achieved through the activation of nucleotide excision repair genes, facilitation of cyclobutane ring repair, inhibition of ROS production, and downregulation of NF-κB and MAPK signaling pathways. The protective properties of propolis extracts are evident in human experimental in vitro skin models, where they effectively inhibit UV-induced photodamage by mitigating the occurrence of DNA strand breaks and enhancing cell viability [120]. For instance, propolis is acknowledged as a powerful inhibitor of tyrosinase, a pivotal enzyme in the melanogenesis pathway [121]. Given that UV irradiation can trigger oxidative stress leading to DNA damage and pigmentation disorders, Stavropoulou et al. examined various samples of Greek propolis. Their evaluation involved assessing free radical scavenging, anticollagenase, and antityrosinase activities to determine the antioxidant and anti-aging attributes of propolis. The study results underscored that samples featuring higher levels of flavonoids showcased superior antioxidant activity and noteworthy collagenase inhibition. In contrast, samples rich in terpenoids showed heightened antityrosinase activity [122]. Compelling evidence supports the roles of propolis and its polyphenolic components in combating cancer by countering events such as elevated DNA damage, mutations, strand breaks, chromosomal instability, deregulated DNA repair machinery, impaired cell cycle checkpoints, and dysregulated homologous recombination [123]. Recent research indicates a notable escalation in age-related hepatocellular carcinoma among older individuals [124]. Cigarette smoke-derived carcinogens such as 4-aminobiphenyl (ABP) contribute to the development of hepatocellular carcinoma in humans. The enzyme cytochrome P450 family 2 subfamily E member 1 (CYP2E1), present across mammalian species, can trigger procarcinogens and convert certain specific drugs into cytotoxic products [125]. According to research findings, propolis treatment can inhibit the conversion of the tobacco carcinogen 4-aminobiphenyl (4-ABP) into its metabolite, N-hydroxy-ABP, by inhibiting CYP2E1 expression [126]. This results in reduced production of reactive oxygen species (ROS) and genotoxicity, serving as a protective measure against cancer development. The induction of mutation, cell death, malformation, and cancer is a well-known consequence of ionizing radiation on both somatic and germ cells. Flavonoids are important components of propolis that have neuroprotective effects against brain damage caused by ionizing radiation, attributed in part to their ability to scavenge free radicals and stabilize the DNA double-helix structure [127]. A primary mechanism widely documented to counteract toxic molecules is the removal of reactive oxygen species (ROS) by antioxidants, preventing their interaction with other molecules, including DNA [118]. Studies have confirmed the powerful antioxidant capacity of propolis and its components. The SH-SY5Y cell line is frequently employed as an in vitro experimental model for investigating Parkinson’s disease. A study reported that pretreatment of SH-SY5Y cells with Brazilian green propolis decreases H2O2-generated ROS derived from mitochondria along with a reduction in the signal intensity of 8-oxo-2′-deoxyguanosine (8-oxo-dG lesion is highly mutagenic) [128].
The issue of fertility preservation has gained significant attention in the context of cancer treatment. In a mouse study, in addition to restoring testicular testosterone levels, Indian propolis also alleviated testicular toxicity caused by mitomycin-C by decreasing DNA damage and enhancing antioxidant activity [129]. In alignment with this, a study examining the genotoxicity, cytotoxicity, and clonogenic death of Chinese hamster ovary cells exposed to gamma radiation indicated the potential utility of propolis in mitigating the adverse effects caused by ionizing radiation [130]. It has been observed that apigenin becomes integrated into the nuclear matrix of prostate epithelial cells. Its binding to nucleic acid bases within the matrix is believed to underlie its antioxidant and chemopreventive activities [131].
Iron’s impact on the aging process is frequently underestimated. Despite its importance for living organisms, its reactivity poses a potential risk [132]. With aging, there is a buildup of iron that correlates with the onset of many age-related diseases. Through its strong iron-binding ability and high lipophilicity, caffeic acid phenethyl ester (CAPE), a constituent of propolis, offers protection against cellular DNA damage caused by iron [133]. Studies have demonstrated a correlation between the antioxidant effects of Portuguese propolis extracts and their ability to prevent DNA damage induced by Fe2+ [134].
Diabetes emerges as a key health issue affecting the elderly population. The literature findings suggest that the use of propolis and epigallocatechin gallate results in a significant increase in the survival rate of diabetic mice [135]. Moreover, the administration of this dietary supplementation yields a notable reduction in lipid peroxidation levels in the kidney, liver, and brain tissue, along with reduced DNA damage in the peripheral lymphocytes of diabetic animals [135].
3. Telomere Length
Unique genomic segments called telomeres reside at the extremities of linear chromosomes [136]. Their primary function is to ensure chromosome integrity and genome stability, offering protection against harm and deterioration [137]. Without a telomere maintenance mechanism, telomeres in adult somatic cells undergo a reduction in length after each round of mitotic cell division, ultimately resulting in cellular senescence and aging [138]. The length of telomeres, a complex hereditary characteristic, imposes a significant constraint on telomere function and is intricately tied to the aging process and age-related diseases [139]. Lifestyle stress and environmental factors, such as diet, can influence telomere length [140]. The results of research involving human subjects showed that prolonged and frequent intake of bee products, including propolis, is linked to telomere length [141]. In Nasir et al.’s (2015) study, which involved comparing beekeepers consuming bee products with non-beekeeper control subjects abstaining from bee products and beekeeping-related activities, it was observed that annual bee product consumption correlated with an average increase of 0.258 kbp in telomere length [141]. The results also revealed that a greater daily frequency of bee product intake was associated with an average increment of 2.66 kbp in telomere length [141]. It has been suggested that telomere length might benefit positively from an antioxidant-rich diet, as telomeres are known to be prone to shortening due to ROS-induced damage, as well as being particularly vulnerable to the prevalent lesion 8-oxo-guanine and its accumulation [142].
Quercetin is a major flavonoid compound found in propolis that has gained significant recognition for its antioxidant and anti-inflammatory properties, along with its potential anticancer and antitumor effects [143]. For instance, targeting human telomeric G-quadruplex DNA represents one of the mechanisms through which this propolis component demonstrates its anticancer activity [144]. These findings propose quercetin as a strong contender for telomere targeting and as a potent anticancer agent. Additionally, quercetin acts as a suppressor of telomerase, an enzyme responsible for the maintenance of telomere integrity and length [145,146,147]. Telomerase overexpression, a characteristic feature in almost all human cancers, plays a key role in sustaining telomere length and contributes to abnormal cell proliferation and immortalization [148]. Caffeic acid phenethyl ester (CAPE), another potent bioactive compound found in propolis, also exhibits antitumoral and antioxidant properties, as well as cytotoxic and apoptotic effects. The induction of apoptosis by CAPE has been linked to its ability to induce the activity of the catalytic subunit of telomerase, known as human telomerase reverse transcriptase [149]. In parallel, by specifically targeting leukemia cells derived from leukemia patients, Manisa propolis effectively reduces the expression of human telomerase reverse transcriptase, with chrysin identified as a key constituent responsible for this effect [150,151]. Telomerase activation in cancer has been attributed to several mechanisms, including the involvement of oncogenes like Wnt, which serve as transcriptional regulators of telomerase [148]. The ability of Aydın Turkish propolis to modulate the expression of microRNAs in glioblastoma and brain cancer stem cells underscores its anticancer effect, with the WNT and NOTCH pathways being implicated [152].
This entry is adapted from the peer-reviewed paper 10.3390/cells13050390
This entry is offline, you can click here to edit this entry!
