
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pragya Tiwari | -- | 3456 | 2024-03-04 08:46:02 | | | |
| 2 | Lindsay Dong | Meta information modification | 3456 | 2024-03-04 09:45:59 | | |
Video Upload Options
Plant-microbe associations define a key interaction and have significant ecological and biotechnological perspectives. In recent times, plant-associated microbes from extreme environments have been extensively explored for their multifaceted benefits to plants and the environment, thereby gaining momentum in global research. Plant-associated extremophiles highlight ubiquitous occurrences, inhabiting extreme habitats and exhibiting enormous diversity. The remarkable capacity of extremophiles to exist in extreme environmental conditions is attributed to the evolution of adaptive mechanisms in these microbes at genetic and physiological levels. In addition, the plant-associated extremophiles have a major impact in promoting plant growth and development and conferring stress tolerance to the host plant, thereby contributing immensely to plant adaptation and survival in extreme conditions.
1. Introduction
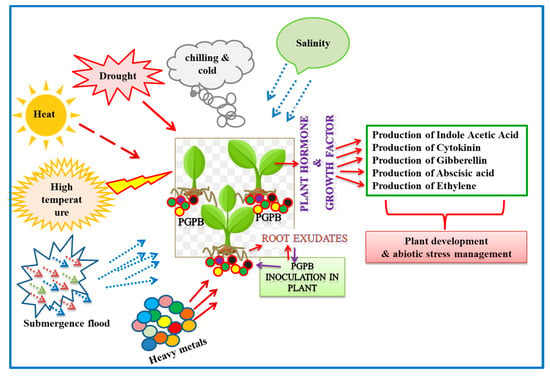
2. Dynamics of Plant-Microbe Interactions in Extreme Habitats
3. Diversity and Distribution of Plant-Associated Extremophiles
3.1. Epiphytic Microbiomes
3.2. Endophytic Microbiomes
3.3. Rhizospheric Microbiomes
4. Plant-Microbe Interactions in Extreme Ecological Habitats
4.1. Acidic Environment
4.2. Alkaline Environment
4.3. Drought Condition
4.4. High Temperature
4.5. Low Temperature
4.6. Saline Condition
4.7. Presence of Heavy Metals
4.8. Flooding Condition
5. Biotechnological Applications of Plant Microbiome
5.1. Plant Growth Promotion
5.1.1. Production of Phytohormones
5.1.2. Biological Nitrogen Fixation
5.1.3. Mineral Solubilization
5.1.4. Biocontrol Function
5.2. Mitigation of Multiple Abiotic Stress
5.2.1. Heat Stress
5.2.2. Cold Stress
5.2.3. Drought Stress
5.2.4. Salinity Stress
References
- Bui, E.N. Soil salinity: A neglected factor in plant ecology and biogeography. J. Arid. Environ. 2013, 92, 14–25.
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163.
- Shu, W.S.; Huang, L.N. Microbial diversity in extreme environments. Nat. Rev. Microbiol. 2022, 20, 219–235.
- Singh, B.K.; Delgado-Baquerizo, M.; Egidi, E.; Guirado, E.; Leach, J.E.; Liu, H.; Trivedi, P. Climate change impacts on plant pathogens, food security, and paths forward. Nat. Rev. Microbiol. 2023, 21, 640–656.
- Dance, A. Studying life at the extremes. Nat. Vol. 2020, 587, 165–166.
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244.
- Talib, K.M.; Luhuai, J.; Chen, X.; Akbar, A.; Tahir, A.; Iqbal, I.; Ali, I. Isolation, culture, and maintenance of Extremophilic fungi. In Extremophilic Fungi; Sahay, S., Ed.; Springer: Singapore, 2022.
- Uzilday, R.O.; Ganie, S.A. Editorial: Extremophiles: Tolerance mechanisms and use in crop improvement. Front. Plant Sci. 2023, 14, 1233202.
- Tiwari, P.; Bae, H. Trends in Harnessing Plant Endophytic microbiome for mitigation of heavy metal toxicity in Plants-a perspective. Plants 2023, 12, 1515.
- Tiwari, P.; Kang, S.; Bae, H. Plant-endophyte associations, rich yet under-explored sources for novel bioactive compounds and applications. Microbiol. Res. 2023, 266, 127241.
- Bose, S.K.; Bajpai, M.; Das, J.; Gautam, A.; Tiwari, P. Actinomycetes endophytes: Overview and significance in the production of bioactive compounds. In Endophytes: Types, Potential Uses, and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: Hauppauge, NY, USA, 2022; ISBN 979-8-88697-045-6.
- Roy, B.; Maitra, D.; Ghosh, J.; Mitra, A.K. Unique extremophilic Bacillus: Their application in plant growth promotion and sustainable agriculture. In Microbes and Microbial Biotechnology for Green Remediation; Malik, J.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 287–304.
- Adil, M.; Tiwari, P.; Chen, J.T.; Khan, R.N.; Kanwal, S. Major bioactive metabolites and antimicrobial potential of Orchidaceae Fungal endophytes. In Advances in Orchid Biology, Biotechnology, and Omics; Tiwari, P., Chen, J., Eds.; Springer Publishers: Berlin/Heidelberg, Germany, 2023; ISBN 13-978-9819910786.
- Dlamini, S.P.; Akanmu, A.O.; Babalola, O.O. Rhizospheric microorganisms: The gateway to sustainable plant health. Front. Sustain. Food Syst. 2022, 6, 925802.
- Lavania, M.; Chauhan, P.S.; Chauhan, S.V.S.; Singh, H.B.; Nautiyal, C.S. Induction of plant defense enzymes and phenolics by treatment with plant growth–promoting rhizobacteria Serratia marcescens NBRI1213. Curr. Microbiol. 2006, 52, 363–368.
- Nutaratat, P.; Srisuk, N.; Arunrattiyakorn, P.; Limtong, S. Plant growth-promoting traits of epiphytic and endophytic yeasts isolated from rice and sugar cane leaves in Thailand. Fungal Biol. 2014, 118, 683–694.
- Tiwari, P. Endophytes: Types, Potential Uses, and Mechanisms of Action; Nova Publishers: Hauppauge, NY, USA, 2022; ISBN 979-8-88697-045-6.
- Ryan, R.P.; Germaine, K.; Franks, A.; Ryan, D.J.; Dowling, D.N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 2008, 278, 1–9.
- Tiwari, P.; Muhammad, A.; Bae, H. Endophyte-mediated bioremediation–an efficient biological strategy in ecological subsistence and agriculture. In Endophytic and Arbuscular Mycorrhizal Fungi and Their Role in Sustainable Agriculture; Erwin, D.J., Ed.; Nova Publishers: Hauppauge, NY, USA, 2023; ISBN 979-8-88697-766-0.
- Tiwari, P.; Mishra, B.N.; Sangwan, N.S. β-glucosidases from the fungus Trichoderma: Efficient cellulose machinery in biotechnological application. BioMed. Res. Int. 2013, 2023, 203735.
- Tiwari, P.; Dufosse, L. Focus and insights into the synthetic biology-mediated chassis of economically important fungi for the production of high-value metabolites. Microorganisms 2023, 11, 1141.
- Rucker, H.R.; Kaçar, B. Enigmatic evolution of microbial nitrogen fixation: Insights from Earth’s past. Trends Microbiol. 2023, 23, 91–94.
- Saeed, Q.; Xiukang, W.; Haider, F.U.; Kučerik, J.; Mumtaz, M.Z.; Holatko, J.; Naseem, M.; Kintl, A.; Ejaz, M.; Naveed, M.; et al. Rhizosphere bacteria in plant growth promotion, biocontrol, and bioremediation of contaminated sites: A comprehensive review of effects and mechanisms. Int. J. Mol. Sci. 2021, 22, 10529.
- Fukami, J.; Cerezini, P.; Hungria, M. Azospirillum: Benefits that go far beyond biological nitrogen fixation. AMB Express 2018, 8, 73.
- Ali, B.; Sabri, A.N.; Ljung, K.; Hasnain, S. Auxin production by plant-associated bacteria: Impact on endogenous IAA content and growth of Triticum aestivum L. Lett. Appl. Microbiol. 2009, 48, 542–547.
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.S.; Krishnani, K.K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L.) on germination and seedling growth of wheat under saline condi-tions. Appl. Biochem. Biotechnol. 2016, 180, 872–882.
- Mukhtar, S.; Shahid, I.; Mehnaz, S.; Malik, K.A. Assessment of two carrier materials for phosphate solubilizing biofertilizers and their effect on the growth of wheat (Triticum aestivum L.). Microbiol. Res. 2017, 205, 107–117.
- Panlada, T.; Pongdet, P.; Aphakorn, L.; Rujirek, N.N.; Nantakorn, B.; Neung, T. Alleviation of the effect of environmental stresses using co-inoculation of mungbean by Bradyrhizobium and Rhizobacteria containing stress-induced ACC deaminase enzyme. Soil Sci. Plant Nut. 2013, 59, 559–571.
- Mukhtar, S.; Mehnaz, S.; Mirza, M.S.; Mirza, B.S.; Malik, K.A. Diversity of Bacillus-like bacterial community in the rhizospheric and non-rhizospheric soil of halophytes (Salsola stocksii and Atriplex amnicola), and characterization of osmoregulatory genes in halophilic Bacilli. Can. J. Microbiol. 2018, 64, 567–579.
- Barka, A.; Nowak, E.; Clément, C.J. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, Burkholderia phytofirmans strain PsJN. Appl. Environ. Microbiol. 2006, 72, 7246–7252.
- Dastager, S.G.; Deepa, C.K.; Pandey, A. Isolation and characterization of novel plant growth promoting Micrococcus sp. NII-0909 and its interaction with cowpea. Plant Physiol. Biochem. 2010, 48, 987–992.
- Tani, C.; Sasakawa, H.; Takenouchi, K. Isolation of endophytic Frankia from root nodules of Casuarina equisetifolia and infec-tivity of the isolate to host plants. Soil Sci. Plant Nutr. 2003, 49, 137–142.
- Singh, S.; Kumar, V.; Singh, S.; Dhanjal, D.S.; Datta, S.; Singh, J. Global scenario of plant–microbiome for sustainable agriculture: Current advancements and future challenges. In Plant Microbiomes for Sustainable Agriculture, Sustainable Development and Biodiversity; Yadav, A.N., Ed.; Springer Nature: Cham, Switzerland, 2020.
- Rekadwad, B.; Li, W.J.; Gonzalez, J.M.; Devasya, R.P.; Bhagwath, A.A.; Urana, R.; Parwez, K. Extremophiles: The species that evolve and survive under hostile conditions. 3 Biotech 2023, 13, 316.
- Wejse, P.L.; Ingvorsen, K.; Mortensen, K.K. Purification and characterization of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 2003, 7, 423–431.
- Bajpai, M.; Das, J.; Tiwari, P. Molecular and biochemical methods for identification, isolation, and characterization of Endophytes. In Endophytes: Types, Potential Uses, and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: Hauppauge, NY, USA, 2022; ISBN 979-8-88697-045-6.
- Taş, N.; de Jong, A.E.E.; Li, Y.; Trubl, G.; Xue, Y.; Dove, N.C. Metagenomic tools in microbial ecology research. Curr. Opin. Biotechnol. 2021, 67, 184–191.
- Bashir, I.; War, A.F.; Rafiq, I.; Reshi, Z.A.; Rashid, I.; Shouche, Y.S. Phyllosphere microbiome: Diversity and functions. Microbiol. Res. 2022, 254, 126888.
- Remus-Emsermann, M.N.P.; Lücker, S.; Müller, D.B.; Potthoff, E.; Daims, H.; Vorholt, J.A. Spatial distribution analyses of natural phyllosphere-colonizing bacteria on Arabidopsis thaliana revealed by fluorescence in situ hybridization. Environ. Microbiol. 2014, 16, 2329–2340.
- Schlechter, R.O.; Miebach, M.; Remus-Emsermann, M.N.P. Driving factors of epiphytic bacterial communities: A review. J. Adv. Res. 2019, 19, 57–65.
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883.
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663.
- Sridhar, K.R. Diversity, ecology, and significance of fungal endophytes. In Endophytes and Secondary Metabolites; Jha, S., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 61–100.
- Tiwari, P.; Bae, H. Horizontal gene transfer and Endophytes: An implication for the acquisition of novel traits. Plants 2020, 9, 305.
- Hata, K.; Sone, K. Isolation of endophytes from leaves of Neolitsea sericin broadleaf and conifer stands. Mycoscience 2008, 49, 229–232.
- Ganley, R.J.; Newcombe, G. Fungal endophytes in seeds and needles of Pinus monticola. Mycol. Res. 2006, 110, 318–327.
- Rai, R.P.; Dash, P.K.; Prasanna, B.M.; Singh, A. Endophytic bacterial flora in the stem tissue of tropical maize (Zea mays L.) genotype: Isolation, identification, and enumeration. World J. Microbiol. Biotechnol. 2007, 23, 853–858.
- Rungjindamai, N.; Pinruan, U.; Choeyklin, R.; Hattori, T.; Jones, E.B.G. Molecular characterization of Basidiomycetous endophytes isolated from leaves, rachis, and petioles of the oil palm, Elaeis guineensis, in Thailand. Fungal Divers. 2008, 33, 139–161.
- Bezerra, J.D.P.; Santos, M.G.S.; Svedese, V.M.; Lima, D.M.; Fernandes, M.J.; Paiva, L.M.; Souza-Motta, C.M. Richness of endophytic fungi isolated from Opuntia ficus-indica Mill. (Cactaceae) and preliminary screening for enzyme production. World J. Microbiol. Biotechnol. 2012, 28, 1989–1995.
- Tiwari, P.; Srivastava, Y.; Bae, H. Trends of pharmaceutical design of Endophytes as anti-infective. Curr. Top. Med. Chem. 2021, 21, 1572–1586.
- Tiwari, P.; Mohd, A.; Basavegowda, N.; Chen, J. Plant-associated endophytes: Molecular mechanisms and significance in promoting sustainable agriculture. In Endophytes: Types, Potential Uses, and Mechanisms of Action; Tiwari, P., Ed.; Nova Publishers: Hauppauge, NY, USA, 2022; ISBN 979-8-88697-045-6.
- Tiwari, P.; Bae, H. Endophytic fungi: Insights, prospects, and challenges in natural product drug discovery. Microorganisms 2022, 10, 360.
- Farrar, K.; Bryant, D.; Cope-Selby, N. Understanding and engineering beneficial plant–microbe interactions: Plant growth pro motion in energy crops. Plant Biotechnol. J. 2014, 12, 1193–1206.
- Mukhtar, S.; Mehnaz, S.; Malik, K.A. Microbial diversity in the rhizosphere of plants growing under extreme environments and its impact on crop improvement. Environ. Sustain. 2019, 2, 329–338.
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506.
- Gómez, F. Acidophile. In Encyclopedia of Astrobiology; Gargaud, M., Amils, R., Quintanilla, J.C., Cleaves, H.J., Irvine, W.M., Pinti, D.L., Viso, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2011.
- Feliatra, F.; Lukistyowati, I.; Yoswaty, D.; Rerian, H.; Melina, D.; Hasyim, W.; Nugroho, T.T.; Fauzi, A.R.; Yolanda, R. Phylogenetic analysis to compare populations of acid-tolerant bacteria isolated from the gastrointestinal tract of two different prawn species Macrobrachium rosenbergii and Penaeus monodon. AACL Bioflux 2016, 9, 360–368.
- Dang, P.; Yu, X.; Le, H.; Liu, J.; Shen, Z.; Zhao, Z. Effects of stand age and soil properties on soil bacterial and fungal community composition in Chinese pine plantations on the Loess Plateau. PLoS ONE 2017, 12, e0186501.
- Madigan, M.; Martinko, J. Brock Biology of Microorganisms, 11th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2005; ISBN 0-13-144329-1.
- Tiwari, P.; Bajpai, M.; Singh, L.K.; Yadav, A.; Bae, H. Portraying fungal mechanisms in stress tolerance: Perspective for sustainable agriculture. In Recent Trends in Mycological Research, Vol 1: Agricultural and Medical Perspective; Yadav, A.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 269–292. ISBN 978-3-030-60658-9.
- Tiwari, P.; Bajpai, M.; Singh, L.K.; Mishra, S.; Yadav, A.N. Phytohormones producing fungal communities: Metabolic engineering for abiotic stress tolerance in plants. In Agriculturally Important Fungi for Sustainable Agriculture; Gupta, V.K., Maria Tuohy, M., Eds.; Springer: Cham, Switzerland, 2020; pp. 171–197. ISBN 978-3-030-45970-3.
- Borkar, S. Alkaliphilic Bacteria: Diversity, Physiology and Industrial Applications. In Bioprospects of Coastal Eubacteria; Borkar, S., Ed.; Springer: Cham, Switzerland, 2015.
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (PGPR) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448.
- Kumar, A.; Verma, J.P. Does plant-microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52.
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C. Simultaneous detection and quantification of indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) produced by rhizobacteria from l-tryptophan (Trp) using HPTLC. J. Microbiol. Method 2015, 110, 7–14.
- Jiang, S.; Zhang, D.; Wang, L.; Pan, J.; Liu, Y.; Kong, X.; Zhou, Y.; Li, D. A maize calcium-dependent protein kinase gene, ZmCPK4, positively regulated abscisic acid signaling and enhanced drought stress tolerance in transgenic Arabidopsis. Plant Physiol. Biochem. 2013, 71, 112–120.
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil 2013, 366, 93–105.
- Vardharajula, S.; Ali, S.Z.; Grover, M.; Reddy, G.; Bandi, V. Drought-tolerant plant growth promoting Bacillus spp.: Effect on growth osmolytes, and antioxidant status of maize under drought stress. J. Plant Interact. 2011, 6, 1–14.
- Naseem, H.; Ahsan, M.; Shahid, M.A.; Khan, N. Exopolysaccharides producing rhizobacteria and their role in plant growth and drought tolerance. J. Basic Microbiol. 2018, 58, 1009–1022.
- Omae, N.; Tsuda, K. Plant-microbiota interactions in abiotic stress environments. MPMI 2022, 35, 511–526.
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331.
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225246.
- Xu, Z.; Shimizu, H.; Ito, S.; Yagasaki, Y.; Zou, C.; Zhou, G.; Zheng, Y. Effects of elevated CO2, warming and precipitation change on plant growth, photosynthesis, and peroxidation in dominant species from North China grassland. Planta 2014, 239, 421–435.
- Mukhtar, S.; Ishaq, A.; Hassan, S.; Mehnaz, S.; Mirza, M.S.; Malik, K.A. Comparison of microbial communities associated with halophyte (Salsola stocksii) and non-halophyte (Triticum aestivum) using culture-independent approaches. Pol. J. Microbiol. 2017, 66, 375–386.
- Maijala, P.; Kango, N.; Szijarto, N.; Viikari, L. Characterization of hemicellulases from thermophilic fungi. Antonie Van Leeuwenhoek 2012, 101, 905–917.
- de Cassia Pereira, J.; Paganini Marques, N.; Rodrigues, A.; Brito de Oliveira, T.; Boscolo, M.; Da Silva, R.; Gomes, E.; Bocchini Martins, D.A. Thermophilic fungi as new sources for the production of cellulases and xylanases with potential use in sugarcane bagasse saccharification. J. Appl. Microbiol. 2015, 118, 928–939.
- Yang, X.; Zhang, J.; Ding, Q.; He, Z.-C.; Zhu, C.Y.; Zhang, K.-Q.; Niu, X.-M. Metabolites from two dominant thermophilic fungal species Thermomyces lanuginosus and Scytalidium thermophilum. Chemi. Biodiver. 2020, 17, e2000137.
- Sandona, K.; Billingsley Tobias, T.L.; Hutchinson, M.I.; Natvig, D.O.; Porras-Alfaro, A. Diversity of thermophilic and thermotolerant fungi in corn grain. Mycologia 2019, 111, 719–729.
- Tiwari, P.; Bose, S.K.; Bae, H. Plant growth promoting soil microbiomes: Beneficial attributes and potential applications. In Soil Microbiomes for Sustainable Agriculture—Volume 2: Functional Annotation; Sustainable Development, and Biodiversity; Yadav, A.N., Ed.; Springer: Cham, Switzerland, 2021; Volume 27, pp. 1–30. ISBN 978-3-030-73506-7.
- Kanekar, P.P.; Kanekar, S.P. Psychrophilic, Psychrotrophic, and Psychrotolerant microorganisms. In Diversity and Biotechnology of Extremophilic Microorganisms from India. Microorganisms for Sustainability; Kanekar, P.P., Kanekar, S.P., Eds.; Springer: Singapore, 2022.
- Yakimov, M.M.; Giuliano, L.; Gentile, G.; Crisafi, E.; Chernikova, T.N.; Abraham, W.-R.; Lunsdorf, H.; Timmis, K.N.; Golyshin, P.N. Oleispira antarctica gen. nov., sp. nov., a novel hydrocarbonoclastic marine bacterium isolated from Antarctic coastal seawater. Int. J. Syst. Evol. Microbiol. 2003, 53, 779–785.
- Humphry, D.R.; George, A.; Black, G.W.; Cummings, S.P. Flavobacterium frigidarium sp. nov., an aerobic, psychrophilic, xylanolytic and laminarinolytic bacterium from Antarctica. Int. J. Syst. Evol. 2001, 51, 1235–1243.
- Gosink, J.; Herwig, R.; Staley, J. Octadecabacter arcticus gen. nov., sp. nov., and O. antarcticus, sp. nov., non-pigmented, psychrophilic gas vacuolate bacteria from polar sea ice and water. Syst. Appl. Microbiol. 1997, 20, 356–365.
- Zhang, D.-C.; Busse, H.-J.; Liu, H.-C.; Zhou, Y.-G.; Schinner, F.; Margesin, R. Sphingomonas glacialis sp. nov., a psychrophilic bacterium isolated from alpine glacier cryoconite. Int. J. Syst. Evol. Microbiol. 2011, 61, 587–591.
- Franzmann, P.; Stackebrandt, E.; Sanderson, K.; Volkman, J.; Cameron, D.; Stevenson, P.; McMeekin, T.; Burton, H. Halobacterium lacusprofundi sp. nov., a halophilic bacterium isolated from Deep Lake, Antarctica. Syst. Appl. Microbiol. 1988, 11, 20–27.
- Bowman, J.P. Description of Cellulophaga algicola sp. nov., isolated from the surfaces of Antarctic algae. Int. J. Syst. Evol. Microbiol. 2000, 50, 1861–1868.
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131.
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm-forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227.
- Bacilio, M.; Moreno, M.; Bashan, Y. Mitigation of negative effects of progressive soil salinity gradients by application of humic acids and inoculation with Pseudomonas stutzeri in a salt-tolerant and a salt-susceptible pepper. Appl. Soil. Ecol. 2016, 107, 394–404.
- Krishnamoorthy, R.; Kim, K.; Subramanian, P.; Senthilkumar, M.; Anandham, R.; Sa, T. Arbuscular mycorrhizal fungi and associated bacteria isolated from salt-affected soil enhance the tolerance of maize to salinity in coastal reclamation soil. Agric. Ecosyst. Environ. 2016, 231, 233–239.
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598.
- Egamberdiyeva, D. The effect of plant growth-promoting bacteria on growth and nutrient uptake of maize in two different soils. Appl. Soil Ecol. 2007, 36, 184–189.
- Bai, J.; Yang, X.; Du, R.; Chen, Y.; Wang, S.; Qiu, R. Biosorption mechanisms involved in immobilization of soil Pb by Bacillus subtilis DBM in a multi-metal contaminated soil. J. Environ. Sci. 2014, 26, 2.
- Khan, Z.; Rehman, A.; Hussain, S.Z.; Nisar, M.A.; Zulfiqar, S.; Shakoori, A.R. Cadmium resistance and uptake by bacterium, Salmonella enterica 43C, isolated from industrial effluent. Amb. Express 2016, 6, 54.
- Zhou, W.; Zhang, H.O.; Ma, Y.; Zhou, J.; Zhang, Y. Bio-removal of cadmium by growing deep-sea bacterium Pseudoalteromonas sp. SCSE709-6. Extremophiles 2013, 17, 723–731.
- Dai, S.; Chen, Q.; Jiang, M.; Wang, B.; Xie, Z.; Yu, N.; Zhou, Y.; Li, S.; Wang, L.; Hua, Y.; et al. Colonized extremophile Deinococcus radiodurans alleviates toxicity of cadmium and lead by suppressing heavy metal accumulation and improving antioxidant system in rice. Environ. Pollut. 2021, 284, 117127.
- Santos, S.P.; Yang, Y.; Rosa, M.T.G.; Rodrigues, M.A.A.; De La Tour, C.B.; Sommer, S.; Teixeira, M.; Carrondo, M.A.; Cloetens, P.; Abreu, I.A.; et al. The interplay between Mn and Fe in Deinococcus radiodurans triggers cellular protection during paraquat-induced oxidative stress. Sci. Rep. 2019, 9, 17217.
- Hobbie, S.E.; Grimm, N.B. Nature-based approaches to managing climate change impacts in cities. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2020, 375, 20190124.
- Martínez-Arias, C.; Witzell, J.; Solla, A.; Martin, J.A.; Rodríguez-Calcerrada, J. Beneficial and pathogenic plant-microbe interactions during flooding stress. Plant Cell Environ. 2022, 45, 2875–2897.
- Waadt, R.; Hsu, P.K.; Schroeder, J.I. Abscisic acid and other plant hormones: Methods to visualize distribution and signaling. Bioessays 2015, 37, 1338–1349.
- Wani, S.H.; Kumar, V.; Shriram, V.; Sah, S.K. Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J. 2016, 4, 162–176.
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172.
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Hontzeas, N.; Davies, W.J. Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. J. Exp. Bot. 2007, 58, 1485–1495.
- Bhadrecha, P.; Singh, S.; Dwibedi, V. A plant’s major strength in the rhizosphere: The plant growth promoting rhizobacteria. Arch. Microbiol. 2023, 205, 165.
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 2104.
- Abd Allah, E.F.; Alqarawi, A.A.; Hashem, A.; Radhakrishnan, R.; Al-Huqail, A.A.; Al-Otibi, F.O.N.; Malikk, J.A.; Alharbi, R.I.; Egamberdieva, D. The endophytic bacterium Bacillus subtilis (BERA 71) improves salt tolerance in chickpea plants by regulating the plant defense mechanisms. J. Plant Interact. 2018, 13, 37–44.
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22.
- Shahzad, R.; Khan, A.L.; Bilal, S.; Waqas, M.; Kang, S.M.; Lee, I.J. Inoculation of abscisic acid-producing endophytic bacteria enhances salinity stress tolerance in Oryza sativa. Environ. Exp. Bot. 2017, 136, 68–77.
- Dekas, A.D.; Poretsky, R.S.; Orphan, V.J. Deep-sea archaea fix and share nitrogen in methane-consuming microbial consortia. Science 2009, 326, 422–426.
- Kuan, K.B.; Othman, R.; Abdul Rahim, K.; Shamsuddin, Z.H. Plant growth promoting rhizobacteria inoculation to enhance vegetative growth, nitrogen fixation and nitrogen remobilization of maize under greenhouse conditions. PLoS ONE 2016, 11, e0152478.
- Hider, R.C.; Kong, X. Chemistry and biology of siderophores. Nat. Prod. Rep. 2010, 27, 637–657.
- Goswami, D.; Thakker, J.N.; Dhandhukia, P.C.; Tejada, M.M. Portraying mechanics of plant growth promoting rhizobacteria (PGPR): A review. Cogent. Food Agric. 2016, 2, 1127500.
- Wang, D.; Lv, S.; Jiang, P.; Li, Y. Roles regulation and agricultural application of plant phosphate transporters. Front. Plant Sci. 2017, 8, 817.
- Oteino, N.; Lally, R.D.; Kiwanuka, S.; Lloyd, A.; Ryan, D.; Germaine, K.J.; Dowling, D.N. Plant growth promotion induced by phosphate solubilizing endophytic Pseudomonas isolates. Front. Microbiol. 2015, 6, 745.
- Joe, M.M.; Devara, S.; Benson, A.; Sa, T. Isolation of phosphate solubilizing endophytic bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of plant growth promotion and antioxidant activity under salt stress. J. Appl. Res. Med. Aromat. Plants 2016, 3, 71–77.
- Singh, M.; Singh, D.; Gupta, A.; Pandey, K.D.; Singh, P.K.; Kumar, A. Plant growth promoting Rhizobacteria: Application in biofertilizers and biocontrol of phytopathogens. In PGPR Amelioration in Sustainable Agriculture; Woodhead Publishing: Sawston, UK, 2019; pp. 41–66.
- Kumar, A.; Vandana, R.S.; Singh, M.; Pandey, K.D. Plant growth promoting rhizobacteria (PGPR). A promising approach for disease management. In Microbes and Environmental Management; Singh, J.S., Singh, D.P., Eds.; Studium Press: New Delhi, India, 2015; pp. 195–209.
- Singh, M.; Kumar, A.; Singh, R.; Pandey, K.D. Endophytic bacteria: A new source of bioactive compounds. 3 Biotech 2017, 7, 315.
- Tapias, D.R.; Galvan, A.M.; Diaz, S.P.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272.
- Sharma, A.; Shankhdhar, D.; Shankhdhar, S.C. Enhancing grain iron content of rice by the application of plant growth promoting rhizobacteria. Plant Soil Environ. 2013, 59, 89–94.
- Sen, S.; Chandrasekhar, C.N. Effect of PGPR on growth promotion of rice (Oryza sativa L.) under salt stress. Asian J. Plant Sci. Res. 2014, 4, 62–67.
- Hey, S.J.; Byrne, E.; Halford, N.G. The interface between metabolic and stress signaling. Ann. Bot. 2010, 105, 197–203.
- Matilla, M.A.; Krell, T. Plant growth promotion and biocontrol mediated by plant-associated bacteria. In Plant Microbiome: Stress Response. Microorganisms for Sustainability; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; Volume 5.
- Souza, R.D.; Ambrosini, A.; Passaglia, L.M.P. Plant growth-promoting bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419.
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 2016, 243, 1251–1264.
- Ortiz, N.; Armadaa, E.; Duque, E.; Roldanc, A.; Azcona, R. Contribution of arbuscular mycorrhizal fungi and/or bacteria to enhancing plant drought tolerance under natural soil conditions: Effectiveness of autochthonous or allochthonous strains. J. Plant Physiol. 2015, 174, 87–96.
- El-Daim, I.A.A.; Bejai, S.; Meijer, J. Improved heat stress tolerance of wheat seedlings by bacterial seed treatment. Plant Soil 2014, 379, 337–350.
- Lamichhane, J.R. Rising risks of late-spring frosts in a changing climate. Nat. Clim. Chang. 2021, 11, 554–555.
- Pareek, A.; Sopory, S.K.; Bohnert, H.K.; Govindjee. Abiotic Stress Adaptation in Plants: Physiological, Molecular and Genomic Foundation; Springer: Dordrecht, The Netherlands, 2010; 526p.
- Miura, K.; Furumoto, T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013, 14, 5312–5337.
- Nagarajan, S.; Nagarajan, S. Abiotic tolerance and crop improvement. In Abiotic Stress Adaptation in Plants; Pareek, A., Sopory, S.K., Bohnert, H., Gobindjee, A., Eds.; Springer: Amsterdam, The Netherlands, 2010; pp. 1–11.
- Su, F.; Jacquard, C.; Villaume, S.; Michel, J.; Rabenoelina, F.; Clement, C.; Barka, E.A.; Dhondt-Cordelier, S.; Vaillant-Gaveau, N. Burkholderia phytofirmans PsJN reduces the impact of freezing temperatures on photosynthesis in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 810.
- Mittler, R. Abiotic stress, the field environment, and stress combination. Trends Plant Sci. 2006, 11, 15–19.
- Franken, P. The plant strengthening root endophyte Piriformospora indica: Potential application and the biology behind. Appl. Microbiol. Biotechnol. 2012, 96, 1455–1464.
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868.
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, A.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221.
- Chang, P.; Gerhardt, K.E.; Huang, X.D.; Yu, X.M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth promoting bacteria facilitate the growth of barley and oats in salt impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremediat. 2014, 16, 1133–1147.
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans PsJN and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39.
- Naveed, M.; Hussain, M.B.; Zahir, Z.A.; Mitter, B.; Sessitsch, A. Drought stress amelioration in wheat through inoculation with Burkholderia phytofirmans strain PsJN. Plant Growth Regul. 2014, 73, 121–131.
- Pinedo, I.; Ledger, T.; Greve, M.; Poupin, M.J. Burkholderia phytofirmans PsJN induces long-term metabolic and transcriptional changes involved in Arabidopsis thaliana salt tolerance. Front. Plant Sci. 2015, 6, 466.




