Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Xinwei Ming | -- | 2633 | 2024-03-04 02:49:06 | | | |
| 2 | Catherine Yang | Meta information modification | 2633 | 2024-03-04 03:18:14 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Ming, X.; Wu, Y.; Zhang, Z.; Li, Y. Micro-Arc Oxidation in Titanium and Its Alloys. Encyclopedia. Available online: https://encyclopedia.pub/entry/55794 (accessed on 07 February 2026).
Ming X, Wu Y, Zhang Z, Li Y. Micro-Arc Oxidation in Titanium and Its Alloys. Encyclopedia. Available at: https://encyclopedia.pub/entry/55794. Accessed February 07, 2026.
Ming, Xinwei, Yan Wu, Ziyue Zhang, Yan Li. "Micro-Arc Oxidation in Titanium and Its Alloys" Encyclopedia, https://encyclopedia.pub/entry/55794 (accessed February 07, 2026).
Ming, X., Wu, Y., Zhang, Z., & Li, Y. (2024, March 04). Micro-Arc Oxidation in Titanium and Its Alloys. In Encyclopedia. https://encyclopedia.pub/entry/55794
Ming, Xinwei, et al. "Micro-Arc Oxidation in Titanium and Its Alloys." Encyclopedia. Web. 04 March, 2024.
Copy Citation
Titanium (Ti) and its alloys are widely recognized as preferred materials for bone implants due to their superior mechanical properties. However, their natural surface bio-inertness can hinder effective tissue integration. To address this challenge, micro-arc oxidation (MAO) has emerged as an innovative electrochemical surface modification technique. Its benefits range from operational simplicity and cost-effectiveness to environmental compatibility and scalability. Furthermore, the distinctive MAO process yields a porous topography that bestows versatile functionalities for biological applications, encompassing osteogenesis, antibacterial, and anti-inflammatory properties.
titanium (Ti)
micro-arc oxidation
surface modification
1. Introduction
The latest statistics show that by mid-century, the global population aged 65 or over is expected to more than double to a staggering 1.6 billion people [1]. An aging population means a larger population base of chronic diseases, and bone and joint diseases. In dentistry and orthopedics, implants, with titanium (Ti) and its alloys as the preferred choice among various materials, have become a common approach for treating conditions due to their exceptional biocompatibility and mechanical properties [2]. Despite the vast potential for Ti and its alloys, there are still limitations to surface bioactivity. They often exhibit biological inertness, limiting the close integration of implants with surrounding tissues. Furthermore, Ti alloys are susceptible to microorganisms, increasing the risk of postoperative infections, and threatening the prognosis of patients. To tackle these issues, apart from alloying [3][4][5], surface treatment proves to be an effective approach as well. Common methodologies encompass atmospheric plasma spraying [6], laser treatment [7], anodizing [8], micro-arc oxidation (MAO), and biomimetic deposition of apatite [9] or other biomaterials [10]. Among the various surface modifications available, MAO has garnered significant attention due to its simplicity of operation, cost-effectiveness, and applicability to complex devices. Through MAO, the surface of Ti-based implants can undergo alterations in microstructural features and chemical composition, rendering them more suitable for tissue adhesion and effectively reducing the risk of infections [11].
2. Applications
MAO coatings, particularly with specific functional additives, possess remarkable biological functionalities including bone promotion, antimicrobial properties, and anti-inflammatory effects. Though clinical applications of Ti-based implants with MAO-modified functional coatings are limited, animal experiments have validated several implants with such coatings (Table 1). Presently, surface modification techniques like sandblasting, acid etching, and anodization, along with the introduction of bioactive materials such as calcium phosphate, hydroxyapatite, and calcium nanoparticles, are widely employed to optimize implant surface microstructure and topography. These modifications primarily aim to enhance biocompatibility and promote osseointegration. For example, MAO techniques have been utilized to create rough and porous surfaces on dental implants and femoral stems, facilitating improved tissue bonding [12].
Table 1. Coatings contain different components with different biological functions and are validated in vitro and in vivo.
| Number | Biologic Function | Adding Substances | Technique | In Vitro | In Vivo | Conclusion/Remark | Reference | |
|---|---|---|---|---|---|---|---|---|
| Cell | Bacterium | Animal Parts | ||||||
| 1 | Osteogenesis | Ca, Sr | MAO | hBMSCs | Simultaneously incorporating Ca and Sr demonstrated superior promotion of hBMSC proliferation. | [13] | ||
| 2 | Osteogenesis/Angiogenesis | Zn | MAO | HUVECs and BMSCs | In the Zn2+ environment, angiogenesis and osteogenesis mutually promote each other. | [14] | ||
| 3 | Osteogenesis/Angiogenesis | Hydroxyapatite nanotubes (HNTs) | MAO | HUVECs and MC3T3-E1 cells | HNT specimens promote both angiogenesis and osteogenesis on cellular and molecular levels. | [15] | ||
| 4 | Osteogenesis | B | MAO, hydrothermal treatment, and heat treatment | SaOS-2 cells | Nanorods inhibit SaOS-2 cell activity, whereas nanoparticles promote it. | [16] | ||
| 5 | Osteogenesis | Hierarchical coatings | MAO, electrochemical reduction | BMSCs | Beagle dogs, the shaft of the canine femur | The hierarchical coatings show higher osteogenesis rates compared to the ordinary MAO group. | [17] | |
| 6 | Osteogenesis | HA, BMP-2 | MAO, dip coating | MC3T3-E1 cells | Beagle femur | The interface bonding strength between HA/BMP-2 coating and surrounding new bone tissue is higher than that of Ca/PMAO coating. | [18] | |
| 7 | Osteogenesis/Angiogenesis | Ca, P, BMP-2 | 3D printing, sandblasting etching, MAO, electrochemical deposition | BMSCs | New Zealand White Rabbit Skull | MAO-CaP-BMP-2 is superior to the MAO and MAO-CaP groups in new bone formation. | [19] | |
| 8 | Osteogenesis/Antibacterial | Ca, P | MAO | hFOBs | E. coli and S. aureus | Volcanic-crater-like and needle-like CaP structures form at 350 V and 450 V, respectively. The former exhibits superior antibacterial performance and biocompatibility. | [20] | |
| 9 | Bioactivity/Antibacterial | Ca, P | MAO, UV catalysis | HGFs | S. sanguinis | Photofunctionalization reduces hydrocarbons and enhances surface protein adsorption. | [21] | |
| 10 | Osteogenesis/Antibacterial | Zn | MAO | MC3T3-E1 cells | E. coli | Incubation with salt solution converts Zn ions into zinc oxide, which helps with long-lasting antibacterial activity. | [22] | |
| 11 | Antibacterial/Osteogenesis/Angiogenesis | Sr, Zn | MAO | HUVECs, BMSC | MRSA and P. gingivalis | Rat femoral model | The surface osteogenesis of samples doped with Sr and Zn is superior to other groups. (No in vivo antibacterial test conducted.) | [23] |
| 12 | Antibacterial | Ag, Cu NPs | MAO | MC3T3-E1 cells | MRSA | Mouse femur ex vivo experiment | Ag and Cu ions synergistically kill bacteria, allowing a 10-fold reduction in Ag ion concentration with consistent antibacterial efficacy. | [24] |
| 13 | Osteogenesis/Antibacterial | Ag, Zn | 3D printing, MAO | MC3T3-E1 cells | MRSA | Mouse femur ex vivo experiment | The synergistic effect of Ag and Zn reduces the concentration of Ag+ by 120 times. | [25] |
| 14 | Osteogenesis/Antibacterial | Ag, Zn | MAO | MC3T3-E1 cells | S. aureus | Ag and ZnO synergy enhances antibacterial performance and promotes CaP phase formation. | [26] | |
| 15 | Osteogenesis/Antibacterial | Ag, Zn | MAO | MC3T3-E1 cells | S. aureus | Ag and Zn ion release is above the antibacterial threshold yet well below cytotoxic levels. | [27] | |
| 16 | Osteogenesis/Antibacterial | Ag, Zn | MAO | S. aureus | Ag and Zn have good synergistic antibacterial effects. | [28] | ||
| 17 | Osteogenesis, Antibacterial | Cu, Zn | MAO | MG63 | E. coli, S. aureus, and MRSA | Orthogonal experiments explore electrolyte effects on coatings, with phytic acid supplying the P element. | [29] | |
| 18 | Skin-integration/Antibacterial | Cu, Zn | MAO | Fibroblasts (L-929) | S. aureus | The synergistic effect of Cu and Zn facilitates skin integration and antibacterial activity. | [30] | |
| 19 | Osteogenesis, Anti-tumor/Antibacterial | Se | MAO | BMSCs, cancerous osteoblasts | S. aureus and E. coli | Se doping enhances osteogenic, anti-tumor, and antibacterial properties. | [31] | |
| 20 | Osteogenesis/Antibacterial | Mn | MAO | MC3T3-E1 cells | E. coli | Rabbit femur | The coating induces osteogenesis and promotes osseointegration. | [32] |
| 21 | Antibacterial | Bi | MAO | MG63 cells | A. actinomycetemcomitans, MRSA | Bismuth nitrate has excellent antibacterial activity compared to bismuth acetate, bismuth gallate, and silver nitrate. | [33] | |
| 22 | Osteogenesis/Antibacterial | Ce | MAO | BMSCs | P. gingivalis, S. aureus | Osteoporotic rat hind legs | Ce-TiO2 coating has excellent antibacterial and anti-inflammatory properties. | [34] |
| 23 | Antibacterial | I | MAO, HT, photocatalysis | BMSCs | S. aureus | Tibial Intramedullary Infection Model of Rats | Under NIR, the coating has good antibacterial and osteogenic properties. | [35] |
| 24 | Antibacterial | I | MAO, electrophoresis | BMSCs | S. aureus and E. coli | The rat osteomyelitis intramedullary nail model | Thirty days after implantation, excellent antimicrobial ability was verified. | [36] |
| 25 | Bioactivity/Antibacterial | B | MAO | ADSCs | S. aureus and P. aeruginosa | Add a small amount of sodium tetraborate to the Ca, P electrolyte system. | [37] | |
| 26 | Osteogenesis/Antibacterial | F | MAO | BMSCs | S. aureus and E. coli | Rabbit femur | Coatings with high F addition showed improved antibacterial and osteogenic abilities. | [38] |
| 27 | Antibacterial/Osteogenesis/Angiogenesis | Sr, Co, and F | MAO | BMSCs | S. aureus and E. coli | Rabbit femur | Sr, Co, and F co-doped coatings induce osteogenesis. | [39] |
| 28 | Osteogenesis/Antibacterial | Mn, F | MAO | BMSCs | S. aureus | Mn and F co-doped coatings show excellent wear and corrosion resistance, along with strong antibacterial properties. | [40] | |
| 29 | Osteogenesis/Antibacterial | Cu, BMP-2 | MAO, dip coating | MC3T3-E1 cells | E. coli, MRSA, Neurospora crassa, and Candida albicans | Mouse craniotomy model | The coating significantly promotes osseointegration. | [41] |
| 30 | Osteogenesis/Antibacterial | Ag, HA | MAO, RF-MS | MC3T3-E1 cells | E. coli | This coating exhibits strong biological activity and antibacterial properties. | [42] | |
| 31 | Bioactivity/Antibacterial | Ag NPs, polylactic acid (PLA) | MAO, electrospinning | MC3T3-E1 cells | S. aureus | PLA ultrafine fibers produced by electrospinning can control the release of silver ions. | [43] | |
| 32 | Osteogenesis/Antibacterial | AgNPs, polydopamine | MAO, dip coating | MG63 cells | S. aureus | New Zealand rabbit subdermal implantation | This coating exhibits strong biological activity and antibacterial properties. | [44] |
| 33 | Osteogenesis/Antibacterial | Polydopamine, cationic antimicrobial peptide LL-3, phospholipid | MAO, dip coating | BMSCs and OBs | S. aureus and E. coli | The coating exhibits good osteogenesis and antibacterial properties. | [45] | |
| 34 | Antibacterial | GO | MAO, EPD | S. aureus and E. coli | Achieves ~80% antibacterial activity against E. coli and 100% against S. aureus. | [46] | ||
| 35 | Antibacterial | rGO, Ag NPs | MAO | MC3T3-E1 cells | MRSA | The coating exhibits good osseogenesis and antibacterial properties. | [47] | |
| 36 | Osteogenesis/Antibacterial | HA, chitosan (CS) | MAO, dip coating | MC3T3-E1 cells | E. coli | Higher usage of CS results in decreased biological performance but improved antimicrobial performance. | [48] | |
| 37 | Osteogenesis/Antibacterial | HA, CS hydrogel containing ciprofloxacin | MAO, HT, chemical grafting | hBMSCs | S. aureus and E. coli | The coating exhibits good osseogenesis and antibacterial properties. | [49] | |
| 38 | Osteogenesis/Antibacterial | BMP-2/CS/HA | MAO, dip coating | MC3T3-E1 cells | E. coli | CS encapsulation sustains BMP-2 release with added antibacterial properties. | [50] | |
| 39 | Antibacterial | Vancomycin | MAO, dip coating | The rabbit osteomyelitis model (infection with MRSA) | In vivo studies demonstrate the potential of this coating to prevent MRSA infection. | [51] | ||
| 40 | Osteogenesis/Antibacterial | Vancomycin | MAO, dip coating, chemical grafting | BMSCs | S. aureus | Rat femur | Functional coatings prevent prosthesis infection and promote bone integration at the interface. | [52] |
| 41 | Antibacterial | Mesoporous silica NPs (MSNs), octenidine (OCT) | Electrophoretic-enhanced MAO | OBs | S. aureus and E. coli | The coating exhibits good osseogenesis and antibacterial properties. | [53] | |
| 42 | Bioactivity/Antibacterial | N, Bi | MAO, photocatalysis | HGFs | Streptococcus sanguinis and Actinomyces nasseri | The coating has bactericidal properties under visible light. | [54] | |
| 43 | Osteogenesis/Antibacterial | MoSe2, CS | MAO, electrospinning, photocatalysis | MC3T3-E1 cells | S. mutans | Rat tibia | Adding MoSe2 significantly enhances TiO2 coating photothermal and photodynamic capabilities. | [55] |
| 44 | Skin-integration/Antibacterial | β-FeOOH, Fe-TiO2 | MAO, HT, photocatalysis | Mouse fibroblasts (L-929) | S. aureus | Mouse skin infection model | The β-FeOOH/FeTiO2 heterojunction prevents bacterial infection under light irradiation. | [56] |
| 45 | Osteogenesis/Anti-inflammatory | Ca, Si | MAO | SaOS-2 cells | The coating inhibits inflammation and induces M2 macrophage polarization. | [57] | ||
| 46 | Antibacterial/Immunoregulation | Cu | MAO | RAW 264.7 macrophages, SaOS-2 cells | S. aureus | Cu boosts macrophage-driven osteogenesis and antibacterial activity in biomaterials. | [58] | |
| 47 | Osteogenesis/Anti-inflammatory | Zn | MAO | RAW264.7 macrophages, BMSCs | The coating shows good osteogenic and anti-inflammatory properties. | [59] | ||
| 48 | Osteogenesis/Anti-inflammatory | Mg | MAO | RAW 264.7 macrophages | Mg acts as an anti-inflammatory agent, inhibiting inflammation and promoting osteogenesis. | [60] | ||
| 49 | Anti-inflammatory | Co | MAO | RAW 264.7 macrophages | Mouse air chamber model | Cobalt-loaded Ti exhibits immune-regulatory effects on macrophages. | [61] | |
| 50 | Osteogenesis/Angiogenesis/Anti-inflammatory | Li | MAO | BMDMs, mouse embryonic cell line (C3H10T1/2), HUVEC | Mouse air-pouch model | Low Li doses effectively regulate immunity, and promote osteogenesis. | [62] | |
| 51 | Osteogenesis/Angiogenesis/Anti-inflammatory | HA | MAO, SHT | MC3T3-E1 cells, human umbilical vein fusion cells, RAW 264.7 cells | Rabbit femur | This coating promotes osteogenesis and angiogenesis, and induces M2 macrophage phenotype. | [63] | |
| 52 | Osteogenesis/Anti-inflammatory | HA | MAO, SHT | MC3T3-E1 cells, endothelial cells, RAW 264.7 cells | Rabbit femur | Nanoparticle-shaped HA is beneficial for osteogenesis, angiogenesis, and immune regulation, whereas nanorod-shaped HA is the opposite. | [64] | |
| 53 | Osteogenesis/Anti-inflammatory | SiO2, ZnPs | MAO, sol-gel | MC3T3-E1 cells | The coating shows good osteogenic and anti-inflammatory properties. | [65] | ||
| 54 | Osteogenesis/Anti-inflammatory | Sr, silk fibroin-based wogonin NPs | MAO, electrochemical deposition, LBL | RAW 264.7 cells, OBs | Osteoporotic rat femur | The coating shows good osteogenic and anti-inflammatory properties. | [66] | |
TiUnite and TiUltra surfaces(Nobel Biocare, Zurich, Switzerland), along with the Ospol implant(OspolAB, Malmö, Sweden) and the M implant(Shinhung, Seoul, Republic of Korea), are all manufactured using the MAO process and are commercially available. The TiUnite surface series by Nobel BiocareTM, introduced in the early 2000s, has undergone continuous refinement and gained worldwide acceptance. These implants, containing 7% phosphorus in the form of titanium phosphate chemical bonds, are fabricated using an electrolyte mixture with phosphorus. A comprehensive review [67] encompassing prospective studies spanning from 2000 to 2016 on the clinical performance of implants featuring the TiUnite surface affirms the high survival rates and favorable maintenance of marginal bone associated with such implants. Incidences of peri-implantitis are notably lower for implants employing the TiUnite surface. Notably, implants with this surface consistently exhibit predictable treatment outcomes across diverse indications. In 2019, Nobel BiocareTM introduced the TiUltra surface series (Figure 1), which features a progressively rough and porous surface texture extending from the implant collar to its tip, exhibiting excellent hydrophilic properties [68]. Moreover, the M implants of ShinhungTM are produced using the MAO method with an electrolyte mixture containing magnesium, resulting in a TiO2 surface containing magnesium (≤9.3%) and phosphorus (≤3%) [69]. Similarly, OspolTM implants are created using the MAO method with an electrolyte mixture containing Ca, leading to the incorporation of calcium ions (less than 11%) within the TiO2 in the form of calcium titanium oxide bonds [70].
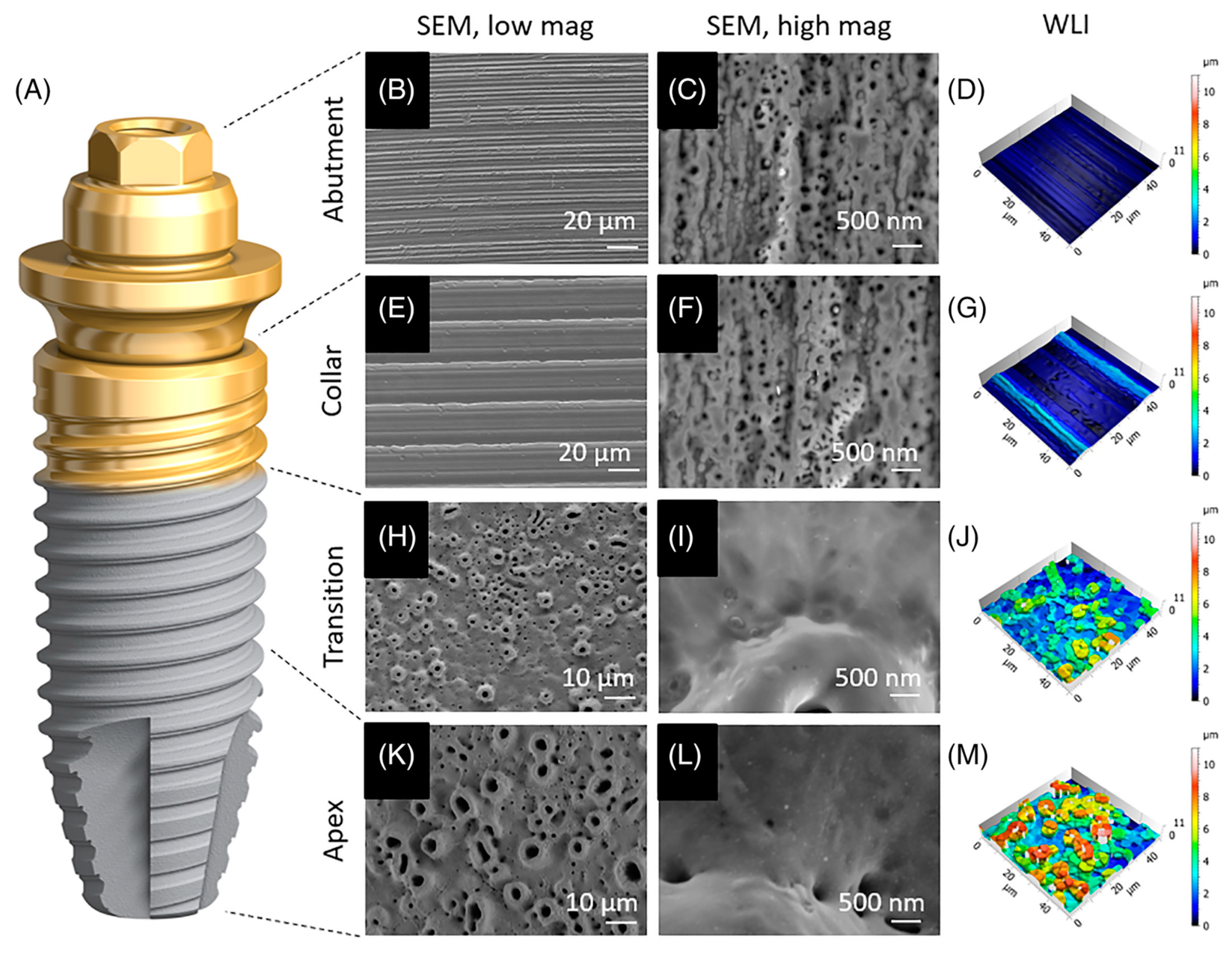
Figure 1. Implants with TiUltra surfaces [68]. (A) Microscopic analysis of the implant system’s four regions: abutment (B–D), implant collar (E–G), transition zone (H–J), and apex (K–M). This includes an overview (B,E,H,K), high-magnification scanning electron micrographs of each region (C,F,I,L), and 3D surface profile reconstructions using white-light interferometry (D,G,J,M).
Femoral shafts treated with sandblasting and MAO coating are commercially available. For example, the Korean BencoxTM hip system (Corentec, Seoul, Republic of Korea) has cementless sandblasted femoral stems with MAO coating on the surface (Figure 2). Made from Ti-6Al-4V alloy, it is a double-wedge straight tapered stem with a rectangular cross-section. The coating method of MAO coating is as follows: After Ti plating, the sample is electrochemically oxidized using the MAO process. The sample is subjected to MAO treatment with a DC pulse power supply in an aqueous electrolyte containing Ca and P.

Figure 2. The BencoxTM stem is a collarless cementless bi-tapered rectangular Ti stem with an MAO-coated sandblasted surface [71].
In a study by Lim et al. [72], in a follow-up period of at least 5 years, the MAO-coated sandblasted surface did not demonstrate superiority over primary total hip arthroplasty (THA) with the same femoral component design. While MAO is theoretically known to promote bone growth through calcium and phosphorus binding to the titanium alloy surface, forming thick oxides and nano-porous coatings, its clinical utility has not been confirmed. In a study with 10 years of follow up [71], 309 cases of THA (involving 256 patients) were performed using the non-cemented BencoxTM hip joint system. A Kaplan–Meier survival analysis indicated a 10-year survival rate of 97.4% when using revision for any reason as the endpoint, and a survival rate of 98.7% when using aseptic loosening revision as the endpoint. While there was a decrease in the proportion of cases with bone resorption in the follow up, the absence of a control group precludes concluding the ability of MAO coatings to resist bone resorption, despite various studies suggesting their potential for preventing bone resorption. In summary, after a follow-up period of at least 10 years, the outcomes of non-cemented sandblasted and MAO-coated THA tapered wedge stems are satisfactory.
The MAO coating cannot only promote bone integration but also improve the wear and corrosion resistance of implants. Khanna et al. [73] performed cold spraying to deposit an Al metal layer onto a Ti-6Al-4V alloy substrate, followed by heat treatment to enhance adhesion. They then conducted MAO treatment to form a dense α-Al2O3 layer on the substrate surface, with a Vickers hardness matching that of sintered alumina used for femoral heads (Figure 3).

Figure 3. Ceramic/metal hybrid artificial hip joint cross-sectional design schematic, with alumina layers formed on Ti alloy for both the cup and head components [73].
3. Challenges
Although MAO coatings have undergone extensive research and led to the emergence of related products, there are still challenges and limitations in the production and application:
-
Despite considerable progress in studying the discharge process of MAO, some micro-scale mechanisms remain unclear, such as cathodic discharge and soft spark discharge. This uncertainty affects the control of the microstructure of MAO coatings [74].
-
In the coating manufacturing process, establishing the electrolyte composition and electrical parameters still necessitates multiple experiments. Defining the optimal parameters remains challenging. Minor losses of electrolytes during usage and the settling of particulate or colloidal electrolytes can also impact coating performance [75][76].
-
The impact of coating morphology on cell bioactivity remains inconclusive. While some studies suggest that moderate roughness aids in cell adhesion and proliferation, and porous surfaces facilitate cell osteogenic differentiation, there is still debate about the optimal pore size. Different cell types may have varied requirements for morphology [77][78]. Additionally, due to cracks and interconnected pores, their durability and wear resistance require attention [79].
-
There is a substantial amount of research on biologically functional coatings; the availability of implants in the market is relatively limited. Successfully translating these technologies into commercial products may face greater challenges [12].
References
- Vereinte, N. (Ed.) Leaving No One Behind in an Ageing World; World social report; United Nations: New York, NY, USA, 2023; ISBN 978-92-1-130458-9.
- Jing, Z.; Zhang, T.; Xiu, P.; Cai, H.; Wei, Q.; Fan, D.; Lin, X.; Song, C.; Liu, Z. Functionalization of 3D-printed titanium alloy orthopedic implants: A literature review. Biomed. Mater. 2020, 15, 052003.
- Jimenez-Marcos, C.; Mirza-Rosca, J.C.; Baltatu, M.S.; Vizureanu, P. Experimental Research on New Developed Titanium Alloys for Biomedical Applications. Bioengineering 2022, 9, 686.
- Zhang, Z.; He, D.; Zheng, Y.; Wu, Y.; Li, Q.; Gong, H.; Ma, X.; Li, Y. Microstructure and mechanical properties of hot-extruded Mg–2Zn-xGa (x = 1, 3, 5 and 7 wt.%) alloys. Mater. Sci. Eng. A 2022, 859, 144208.
- Li, Q.; Ma, X.; Xiong, C.; Qu, W.; Li, Y. Effects of annealing temperature on microstructures and shape memory effect of Ti-19Zr-11Nb-2Ta alloy sheets. J. Alloys Compd. 2022, 897, 162728.
- Istrate, B.; Mareci, D.; Munteanu, C.; Stanciu, S.; Luca, D.; Crimu, C.I.; Kamel, E. In vitro electrochemical properties of biodegradable ZrO2-CaO coated MgCa alloy using atmospheric plasma spraying. J. Optoelectron. Adv. Mater. 2015, 17, 1186–1192.
- Xue, X.; Ma, C.; An, H.; Li, Y.; Guan, Y. Corrosion resistance and cytocompatibility of Ti-20Zr-10Nb-4Ta alloy surface modified by a focused fiber laser. Sci. China Mater. 2018, 61, 516–524.
- Wu, Y.; Li, Q.; Xu, B.; Fu, H.; Li, Y. Nano-hydroxyapatite coated TiO2 nanotubes on Ti-19Zr-10Nb-1Fe alloy promotes osteogenesis in vitro. Colloids Surf. B Biointerfaces 2021, 207, 112019.
- Di Pompo, G.; Liguori, A.; Carlini, M.; Avnet, S.; Boi, M.; Baldini, N.; Focarete, M.L.; Bianchi, M.; Gualandi, C.; Graziani, G. Electrospun fibers coated with nanostructured biomimetic hydroxyapatite: A new platform for regeneration at the bone interfaces. Biomater. Adv. 2023, 144, 213231.
- Nijhuis, A.W.G.; Nejadnik, M.R.; Nudelman, F.; Walboomers, X.F.; Te Riet, J.; Habibovic, P.; Tahmasebi Birgani, Z.; Li, Y.; Bomans, P.H.H.; Jansen, J.A.; et al. Enzymatic pH control for biomimetic deposition of calcium phosphate coatings. Acta Biomater. 2014, 10, 931–939.
- Li, G.; Ma, F.; Liu, P.; Qi, S.; Li, W.; Zhang, K.; Chen, X. Review of micro-arc oxidation of titanium alloys: Mechanism, properties and applications. J. Alloys Compd. 2023, 948, 169773.
- Costa, R.C.; Nagay, B.E.; Dini, C.; Borges, M.H.R.; Miranda, L.F.B.; Cordeiro, J.M.; Souza, J.G.S.; Sukotjo, C.; Cruz, N.C.; Barão, V.A.R. The race for the optimal antimicrobial surface: Perspectives and challenges related to plasma electrolytic oxidation coating for titanium-based implants. Adv. Colloid Interface Sci. 2023, 311, 102805.
- Li, Y.; Wang, W.; Yu, F.; Wang, D.; Guan, S.; Li, Y.; Qi, M. Characterization and cytocompatibility of hierarchical porous TiO2 coatings incorporated with calcium and strontium by one-step micro-arc oxidation. Mater. Sci. Eng. C 2020, 109, 110610.
- Yu, L.; Yin, Y.; Guo, Z.; Fei, Y.; Wen, X.; Wang, J.; Sun, H.; Hu, J.; Jin, S. A functional study of zinc–titanium coatings and exploration of the intrinsic correlation between angiogenesis and osteogenesis. J. Mater. Chem. B 2023, 11, 3236–3251.
- Zhou, Z.; Cai, K.; Shen, J.; Cai, L.; Dai, B.; Wang, Z.; Ma, P.; Liu, J.; Shen, X. Fabrication and biological assessment of halloysite-doped micro/nano structures on titanium surface. Ceram. Int. 2023, 49, 8886–8896.
- Ying, D.; Ouyang, Z.; Liu, T.; Liu, X.; Huang, Q. Boron-containing micro/nano-structured TiO2/bioceramics coatings with modulatory effects on SaOS-2 cell response. Mater. Lett. 2018, 228, 29–32.
- Li, G.; Cao, H.; Zhang, W.; Ding, X.; Yang, G.; Qiao, Y.; Liu, X.; Jiang, X. Enhanced Osseointegration of Hierarchical Micro/Nanotopographic Titanium Fabricated by Microarc Oxidation and Electrochemical Treatment. ACS Appl. Mater. Interfaces 2016, 8, 3840–3852.
- Yu, S.; Yu, Z.; Guo, D.; Zhu, H.; Zhang, M.; Han, J.; Yu, Z.; Cao, Y.; Wang, G. Enhanced bioactivity and interfacial bonding strength of Ti3Zr2Sn3Mo25Nb alloy through graded porosity and surface bioactivation. J. Mater. Sci. Technol. 2022, 100, 137–149.
- Teng, F.-Y.; Tai, I.-C.; Ho, M.-L.; Wang, J.-W.; Weng, L.W.; Wang, Y.J.; Wang, M.-W.; Tseng, C.-C. Controlled release of BMP-2 from titanium with electrodeposition modification enhancing critical size bone formation. Mater. Sci. Eng. C 2019, 105, 109879.
- Alipal, J.; Saidin, S.; Lo, A.Z.K.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. In vitro surface efficacy of CaP-based anodised titanium for bone implants. Surf. Interfaces 2023, 39, 102872.
- Dini, C.; Nagay, B.E.; Cordeiro, J.M.; da Cruz, N.C.; Rangel, E.C.; Ricomini-Filho, A.P.; de Avila, E.D.; Barão, V.A.R. UV-photofunctionalization of a biomimetic coating for dental implants application. Mater. Sci. Eng. C 2020, 110, 110657.
- Shimabukuro, M.; Tsutsumi, Y.; Nozaki, K.; Chen, P.; Yamada, R.; Ashida, M.; Doi, H.; Nagai, A.; Hanawa, T. Chemical and Biological Roles of Zinc in a Porous Titanium Dioxide Layer Formed by Micro-Arc Oxidation. Coatings 2019, 9, 705.
- Yan, R.; Li, J.; Wu, Q.; Zhang, X.; Hu, L.; Deng, Y.; Jiang, R.; Wen, J.; Jiang, X. Trace Element-Augmented Titanium Implant with Targeted Angiogenesis and Enhanced Osseointegration in Osteoporotic Rats. Front. Chem. 2022, 10, 839062.
- van Hengel, I.A.J.; Tierolf, M.W.A.M.; Valerio, V.P.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Self-defending additively manufactured bone implants bearing silver and copper nanoparticles. J. Mater. Chem. B 2020, 8, 1589–1602.
- van Hengel, I.A.J.; Putra, N.E.; Tierolf, M.W.A.M.; Minneboo, M.; Fluit, A.C.; Fratila-Apachitei, L.E.; Apachitei, I.; Zadpoor, A.A. Biofunctionalization of selective laser melted porous titanium using silver and zinc nanoparticles to prevent infections by antibiotic-resistant bacteria. Acta Biomater. 2020, 107, 325–337.
- Lv, Y.; Zhang, T.; Zhang, X.; Fu, S.; Yang, L.; Dong, Z.; Ma, Y.; Zhang, E. The synergistic effect of Ag and ZnO on the microstructure, corrosion resistance and in vitro biological performance of titania coating. Surf. Coat. Technol. 2021, 426, 127798.
- Zhang, L.; Gao, Q.; Han, Y. Zn and Ag Co-doped Anti-microbial TiO2 Coatings on Ti by Micro-arc Oxidation. J. Mater. Sci. Technol. 2016, 32, 919–924.
- Lv, Y.; Cai, G.; Zhang, X.; Fu, S.; Zhang, E.; Yang, L.; Xiao, J.; Dong, Z. Microstructural characterization and in vitro biological performances of Ag, Zn co-incorporated TiO2 coating. Ceram. Int. 2020, 46, 29160–29172.
- Wang, Y.; Zhao, S.; Li, G.; Zhang, S.; Zhao, R.; Dong, A.; Zhang, R. Preparation and in vitro antibacterial properties of anodic coatings co-doped with Cu, Zn, and P on a Ti–6Al–4V alloy. Mater. Chem. Phys. 2020, 241, 122360.
- Zhang, L.; Guo, J.; Yan, T.; Han, Y. Fibroblast responses and antibacterial activity of Cu and Zn co-doped TiO2 for percutaneous implants. Appl. Surf. Sci. 2018, 434, 633–642.
- Zhou, J.; Wang, X. The osteogenic, anti-oncogenic and antibacterial activities of selenium-doped titanium dioxide coatings on titanium. Surf. Coat. Technol. 2020, 403, 126408.
- Zhao, Q.-M.; Sun, Y.-Y.; Wu, C.-S.; Yang, J.; Bao, G.-F.; Cui, Z.-M. Enhanced osteogenic activity and antibacterial ability of manganese–titanium dioxide microporous coating on titanium surfaces. Nanotoxicology 2020, 14, 289–309.
- Lin, D.-J.; Tsai, M.-T.; Shieh, T.-M.; Huang, H.-L.; Hsu, J.-T.; Ko, Y.-C.; Fuh, L.-J. In vitro antibacterial activity and cytocompatibility of bismuth doped micro-arc oxidized titanium. J. Biomater. Appl. 2013, 27, 553–563.
- Qi, S.; Kang, B.; Yao, C.; Lan, D.; Chen, X.; Ma, F.; Liu, P.; Liu, Y. Cerium doped TiO2 coating with superior antibacterial property and biocompatibility prepared by micro-arc oxidation. Mater. Des. 2023, 234, 112312.
- Teng, W.; Zhang, Z.; Wang, Y.; Ye, Y.; Yinwang, E.; Liu, A.; Zhou, X.; Xu, J.; Zhou, C.; Sun, H.; et al. Iodine Immobilized Metal–Organic Framework for NIR-Triggered Antibacterial Therapy on Orthopedic Implants. Small 2021, 17, 2102315.
- Wang, Y.; Teng, W.; Zhang, Z.; Ma, S.; Jin, Z.; Zhou, X.; Ye, Y.; Zhang, C.; Gou, Z.; Yu, X.; et al. Remote Eradication of Bacteria on Orthopedic Implants via Delayed Delivery of Polycaprolactone Stabilized Polyvinylpyrrolidone Iodine. JFB 2022, 13, 195.
- Sopchenski, L.; Cogo, S.; Dias-Ntipanyj, M.F.; Elifio-Espósito, S.; Popat, K.C.; Soares, P. Bioactive and antibacterial boron doped TiO2 coating obtained by PEO. Appl. Surf. Sci. 2018, 458, 49–58.
- Zhou, J.; Li, B.; Han, Y. F-doped TiO2 microporous coating on titanium with enhanced antibacterial and osteogenic activities. Sci. Rep. 2018, 8, 17858.
- Zhou, J.; Zhao, L. Multifunction Sr, Co and F co-doped microporous coating on titanium of antibacterial, angiogenic and osteogenic activities. Sci. Rep. 2016, 6, 29069.
- Zhao, Q.; Wu, J.; Zhang, S.; Ni, X.; Wang, B.; Lu, K.; Zhang, P.; Xu, R. Preparation and properties of composite manganese/fluorine coatings on metallic titanium. RSC Adv. 2023, 13, 14863–14877.
- Popova, A.D.; Sheveyko, A.N.; Kuptsov, K.A.; Advakhova, D.Y.; Karyagina, A.S.; Gromov, A.V.; Krivozubov, M.S.; Orlova, P.A.; Volkov, A.V.; Slukin, P.V.; et al. Osteoconductive, Osteogenic, and Antipathogenic Plasma Electrolytic Oxidation Coatings on Titanium Implants with BMP-2. ACS Appl. Mater. Interfaces 2023, 15, 37274–37289.
- Arun, S.; Ahn, S.-G.; Choe, H.-C. Surface characteristics of HA-coated and PEO-treated Ti-6Al-4V alloy in solution containing Ag nanoparticles. Surf. Interfaces 2023, 39, 102932.
- Zaniolo, K.; Biaggio, S.; Cirelli, J.; Cominotte, M.; Bocchi, N.; Rocha-Filho, R.; De Souza, C. Production and Characterization of Bioactive and Antimicrobial Titanium Oxide Surfaces with Silver Nanoparticles and a Poly (lactic Acid) Microfiber Coating. J. Braz. Chem. Soc. 2023, e-20230134.
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired anchoring AgNPs onto micro-nanoporous TiO2 orthopedic coatings: Trap-killing of bacteria, surface-regulated osteoblast functions and host responses. Biomaterials 2016, 75, 203–222.
- He, Y.; Zhang, Y.; Shen, X.; Tao, B.; Liu, J.; Yuan, Z.; Cai, K. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf. B Biointerfaces 2018, 170, 54–63.
- Mazinani, A.; Nine, M.J.; Chiesa, R.; Candiani, G.; Tarsini, P.; Tung, T.T.; Losic, D. Graphene oxide (GO) decorated on multi-structured porous titania fabricated by plasma electrolytic oxidation (PEO) for enhanced antibacterial performance. Mater. Des. 2021, 200, 109443.
- San, H.; Paresoglou, M.; Minneboo, M.; van Hengel, I.A.J.; Yilmaz, A.; Gonzalez-Garcia, Y.; Fluit, A.C.; Hagedoorn, P.-L.; Fratila-Apachitei, L.E.; Apachitei, I.; et al. Fighting Antibiotic-Resistant Bacterial Infections by Surface Biofunctionalization of 3D-Printed Porous Titanium Implants with Reduced Graphene Oxide and Silver Nanoparticles. Int. J. Mol. Sci. 2022, 23, 9204.
- Li, B.; Xia, X.; Guo, M.; Jiang, Y.; Li, Y.; Zhang, Z.; Liu, S.; Li, H.; Liang, C.; Wang, H. Biological and antibacterial properties of the micro-nanostructured hydroxyapatite/chitosan coating on titanium. Sci. Rep. 2019, 9, 14052.
- Zhou, R.; Zhou, Y.; Cheng, J.; Cao, J.; Li, M.; Yu, H.; Wei, D.; Li, B.; Wang, Y.; Zhou, Y. Surface configuration of microarc oxidized Ti with regionally loaded chitosan hydrogel containing ciprofloxacin for improving biological performance. Mater. Today Bio 2022, 16, 100380.
- Wang, X.; Li, B.; Liu, S.; Zhang, C.; Hao, J. Antibacterial and Biological Properties of a Micro-structured BMP-2/Chitosan/Hydroxyapatite Hybrid Coating on Ti Surface. J. Hard Tissue Biol. 2019, 28, 303–314.
- Zhang, H.; Wang, G.; Liu, P.; Tong, D.; Ding, C.; Zhang, Z.; Xie, Y.; Tang, H.; Ji, F. Vancomycin-loaded titanium coatings with an interconnected micro-patterned structure for prophylaxis of infections: An in vivo study. RSC Adv. 2018, 8, 9223–9231.
- Xu, X.; Xu, H.; Chai, Q.; Li, Z.; Man, Z.; Li, W. Novel functionalized Ti6Al4V scaffold for preventing infection and promoting rapid osseointegration. Mater. Des. 2023, 226, 111612.
- Xu, G.; Shen, X.; Dai, L.; Ran, Q.; Ma, P.; Cai, K. Reduced bacteria adhesion on octenidine loaded mesoporous silica nanoparticles coating on titanium substrates. Mater. Sci. Eng. C 2017, 70, 386–395.
- Nagay, B.E.; Dini, C.; Cordeiro, J.M.; Ricomini-Filho, A.P.; de Avila, E.D.; Rangel, E.C.; da Cruz, N.C.; Barão, V.A.R. Visible-Light-Induced Photocatalytic and Antibacterial Activity of TiO2 Codoped with Nitrogen and Bismuth: New Perspectives to Control Implant-Biofilm-Related Diseases. ACS Appl. Mater. Interfaces 2019, 11, 18186–18202.
- Chai, M.; An, M.; Zhang, X. Construction of a TiO2/MoSe2/CHI coating on dental implants for combating Streptococcus mutans infection. Mater. Sci. Eng. C 2021, 129, 112416.
- Li, K.; Xue, Y.; Zhang, L.; Han, Y. β-FeOOH/Fe-TiO2 heterojunctions on Ti for bacteria inactivation under light irradiation and biosealing. Biomater. Sci. 2020, 8, 6004–6016.
- Huang, Q.; Li, X.; Elkhooly, T.A.; Xu, S.; Liu, X.; Feng, Q.; Wu, H.; Liu, Y. The osteogenic, inflammatory and osteo-immunomodulatory performances of biomedical Ti-Ta metal–metal composite with Ca- and Si-containing bioceramic coatings. Colloids Surf. B Biointerfaces 2018, 169, 49–59.
- Huang, Q.; Li, X.; Elkhooly, T.A.; Liu, X.; Zhang, R.; Wu, H.; Feng, Q.; Liu, Y. The Cu-containing TiO2 coatings with modulatory effects on macrophage polarization and bactericidal capacity prepared by micro-arc oxidation on titanium substrates. Colloids Surf. B Biointerfaces 2018, 170, 242–250.
- Sun, H.; Yang, Y.; Yu, L.; Liu, K.; Fei, Y.; Guo, C.; Zhou, Y.; Hu, J.; Shi, L.; Ji, H. Inhibition of Inflammatory Response and Promotion of Osteogenic Activity of Zinc-Doped Micro-Arc Titanium Oxide Coatings. ACS Omega 2022, 7, 14920–14932.
- Li, X.; Huang, Q.; Liu, L.; Zhu, W.; Elkhooly, T.A.; Liu, Y.; Feng, Q.; Li, Q.; Zhou, S.; Liu, Y.; et al. Reduced inflammatory response by incorporating magnesium into porous TiO2 coating on titanium substrate. Colloids Surf. B Biointerfaces 2018, 171, 276–284.
- Yang, X.; Zhang, C.; Zhang, T.; Xiao, J. Cobalt-doped Ti surface promotes immunomodulation. Biomed. Mater. 2022, 17, 025003.
- Peng, F.; Qiu, L.; Yao, M.; Liu, L.; Zheng, Y.; Wu, S.; Ruan, Q.; Liu, X.; Zhang, Y.; Li, M.; et al. A lithium-doped surface inspires immunomodulatory functions for enhanced osteointegration through PI3K/AKT signaling axis regulation. Biomater. Sci. 2021, 9, 8202–8220.
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358.
- Ramenzoni, L.L.; Flückiger, L.B.; Attin, T.; Schmidlin, P.R. Effect of Titanium and Zirconium Oxide Microparticles on Pro-Inflammatory Response in Human Macrophages under Induced Sterile Inflammation: An In Vitro Study. Materials 2021, 14, 4166.
- Wang, D.; Chen, M.-W.; Wei, Y.-J.; Geng, W.-B.; Hu, Y.; Luo, Z.; Cai, K.-Y. Construction of Wogonin Nanoparticle-Containing Strontium-Doped Nanoporous Structure on Titanium Surface to Promote Osteoporosis Fracture Repair. Adv. Healthc. Mater. 2022, 11, 2201405.
- Wang, X.; Mei, L.; Jiang, X.; Jin, M.; Xu, Y.; Li, J.; Li, X.; Meng, Z.; Zhu, J.; Wu, F. Hydroxyapatite-Coated Titanium by Micro-Arc Oxidation and Steam–Hydrothermal Treatment Promotes Osseointegration. Front. Bioeng. Biotechnol. 2021, 9, 625877.
- Karl, M.; Albrektsson, T. Clinical Performance of Dental Implants with a Moderately Rough (TiUnite) Surface: A Meta-Analysis of Prospective Clinical Studies. Int. J. Oral. Maxillofac. Implants 2017, 32, 717–734.
- Milleret, V.; Lienemann, P.S.; Gasser, A.; Bauer, S.; Ehrbar, M.; Wennerberg, A. Rational design and in vitro characterization of novel dental implant and abutment surfaces for balancing clinical and biological needs. Clin. Implant Dent. Relat. Res. 2019, 21, 15–24.
- Pang, K.-M.; Lee, J.-W.; Lee, J.-Y.; Lee, J.-B.; Kim, S.-M.; Kim, M.-J.; Lee, J.-H. Clinical outcomes of magnesium-incorporated oxidised implants: A randomised double-blind clinical trial. Clin. Oral. Implants Res. 2014, 25, 616–621.
- Kang, B.-S. On the Bone Tissue Response to Surface Chemistry Modifications of Titanium Implants; Department of Biomaterials, Institute of Clinical Sciences, Sahlgrenska Academy at University of Gothenburg: Göteborg, Sweden, 2011; ISBN 978-91-628-8325-6.
- Lim, Y.-W.; Song, J.-H.; Kwon, S.-Y.; Kim, Y.-S.; Byun, Y.-S.; Lee, S.-W. Minimum 10-year follow-up of micro-arc oxidation coating on a cementless grit-blasted tapered-wedge stem of total hip arthroplasty: A multicentre study. HIP Int. 2022, 32, 501–509.
- Lim, Y.W.; Kwon, S.Y.; Sun, D.H.; Kim, Y.S. The Otto Aufranc Award: Enhanced Biocompatibility of Stainless Steel Implants by Titanium Coating and Microarc Oxidation. Clin. Orthop. Relat. Res. 2011, 469, 330–338.
- Khanna, R.; Kokubo, T.; Matsushita, T.; Takadama, H. Fabrication of dense α-alumina layer on Ti-6Al-4V alloy hybrid for bearing surfaces of artificial hip joint. Mater. Sci. Eng. C 2016, 69, 1229–1239.
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Progress Mater. Sci. 2021, 117, 100735.
- Martin, J.; Leone, P.; Nominé, A.; Veys-Renaux, D.; Henrion, G.; Belmonte, T. Influence of electrolyte ageing on the Plasma Electrolytic Oxidation of aluminium. Surf. Coat. Technol. 2015, 269, 36–46.
- Lu, X.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Insights into plasma electrolytic oxidation treatment with particle addition. Corros. Sci. 2015, 101, 201–207.
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2022, 7, e10239.
- Balshe, A.A.; Assad, D.A.; Eckert, S.E.; Koka, S.; Weaver, A.L. A Retrospective Study of the Survival of Smooth- and Rough-Surface Dental Implants. Int. J. Oral Maxillofac. Implant 2009, 24, 1113–1118.
- Aliofkhazraei, M.; Macdonald, D.D.; Matykina, E.; Parfenov, E.V.; Egorkin, V.S.; Curran, J.A.; Troughton, S.C.; Sinebryukhov, S.L.; Gnedenkov, S.V.; Lampke, T.; et al. Review of plasma electrolytic oxidation of titanium substrates: Mechanism, properties, applications and limitations. Appl. Surf. Sci. Adv. 2021, 5, 100121.
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22.
More
Information
Subjects:
Engineering, Biomedical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.1K
Revisions:
2 times
(View History)
Update Date:
04 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




