
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Malgorzata Woronkowicz | -- | 2478 | 2024-03-03 22:58:12 | | | |
| 2 | Mona Zou | Meta information modification | 2478 | 2024-03-04 08:57:16 | | |
Video Upload Options
The corneal epithelium, comprising three layers of cells, represents the outermost portion of the eye and functions as a vital protective barrier while concurrently serving as a critical refractive structure. Maintaining its homeostasis involves a complex regenerative process facilitated by the functions of the lacrimal gland, tear film, and corneal nerves. Crucially, limbal epithelial stem cells located in the limbus (transitional zone between the cornea and the conjunctiva) are instrumental for the corneal epithelium integrity by replenishing and renewing cells. Re-epithelialization failure results in persistent defects, often associated with various ocular conditions including diabetic keratopathy. The insulin-like growth factor (IGF) system is a sophisticated network of insulin and other proteins essential for numerous physiological processes.
1. Introduction
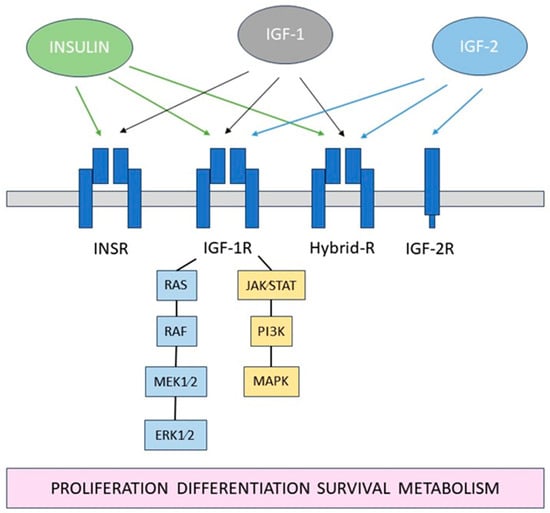
2. The Role of Insulin and Effect of Diabetes
| Receptor | Localization | Ref. |
|---|---|---|
| Insulin receptor | Plasma membrane and cytoplasm; mainly in the wing and superficial cell layers | [6] |
| Nucleus | [7] | |
| Mitochondria | [8] | |
| IGF-1R | All layers of the cornea; mainly around cellular nuclei of actively differentiating epithelial cells | [7] |
| Plasma membrane and cytoplasm | [6] | |
| Mitochondria | [8] | |
| Hybrid- R | Plasma membrane and nucleus | [9] |
| IGF-2R | Central and peripheral epithelium with higher expression in the periphery following corneal injury | [10] |
| Primarily in the basal corneal epithelium in murine and porcine corneas | [11] |
| Ref. | Study Design | Diagnosis | No. of Eyes | Mean Age (Years) |
Eyes with Complete Epithelialization (%) | Mean Time to Epithelialization (Days) |
|---|---|---|---|---|---|---|
| [74] | Randomized controlled trial | Postoperative corneal epithelial defect after vitreoretinal surgery in diabetics | A—8 B—8 C—8 |
A—62.62 ± 5.99 B—56.12 ± 7.77 C—55.75 ± 6.64 |
A—100 B—100 C—100 |
All eyes healed within 6 days 100% eyes in A, 75% eyes in B and 62.5% eyes in C group healed within 3 days. |
| [75] | Randomized clinical trial | Postoperative corneal epithelial defects after vitreoretinal surgery | 19 | 57.05 ± 12.33 | 100 | 3 |
| [76] | Prospective interventional, single-center case series | Refractory persistent epithelial defects | 11 | 45.4 ± 25 | 82 | 62.3 ± 34.6 |
| [77] | Prospective non-randomized hospital-based study | Refractory persistent epithelial defects | 21 | 72.2 | 81 | 34.8 ± 29.9 |
| [78] | Prospective non-randomized hospital-based study | Recurrent epithelial erosions | 15 | 29.00 ± 8.72 | 100 | 21 |
| [79] | Retrospective, observational | Refractory neurotrophic keratopathy (NK) in stages 2 and 3 | 21 | 61 | 90 | 18 ± 9 in NK stage 2; 29 ± 11 in NK stage 3 |
| [80] | Retrospective, consecutive case–control series | Refractory persistent epithelial defects | 61 | 71.5 ± 19.3 | 84 | 32.6 ± 28.3 |
| [81] | Retrospective case series |
Refractory neurotrophic corneal ulcers | 6 | 36.5 | 100 | 7 to 25 |
| [82] | Retrospective Case series |
Corneal epithelial erosions induced during vitreoretinal surgery in diabetics | 5 | 49 | 100 | 2.5 ± 0.6 |
| [83] | Retrospective case series | Dry eye disease | 32 | 61.3 ± 16.8 | - | - |
| [84] | Case report | Corneal ulcer following chemical injury | 1 | 41 | 100 | 60 |
| [85] | Case report | Bilateral Neurotrophic keratitis | 2 | 55 | 100 | 7 |
| [86] | Case report | Neurotrophic keratopathy after resection of acoustic neuroma | 1 | 45 | 100 | 14 |
| [87] | Case report | Neurotrophic keratopathy | 1 | 40 | 100 | 20 |
| [88] | Case report | Neurotrophic keratopathy | 1 | 64 | 100 | 30 |
References
- Vaidyanathan, U.; Hopping, G.C.; Liu, H.Y.; Somani, A.N.; Ronquillo, Y.C.; Hoopes, P.C.; Moshirfar, M. Persistent Corneal Epithelial Defects: A Review Article. Med. Hypothesis Discov. Innov. Ophthalmol. J. 2019, 8, 163–176.
- Tarvestad-Laise, K.E.; Ceresa, B.P. Modulating Growth Factor Receptor Signaling to Promote Corneal Epithelial Homeostasis. Cells 2023, 12, 2730.
- Stuard, W.L.; Titone, R.; Robertson, D.M. IGFBP-3 Functions as a Molecular Switch That Mediates Mitochondrial and Metabolic Homeostasis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2022, 36, e22062.
- Stuard, W.L.; Titone, R.; Robertson, D.M. The IGF/Insulin-IGFBP Axis in Corneal Development, Wound Healing, and Disease. Front. Endocrinol. 2020, 11, 24.
- Naeser, P. Insulin Receptors in Human Ocular Tissues. Immunohistochemical Demonstration in Normal and Diabetic Eyes. Ups. J. Med. Sci. 1997, 102, 35–40.
- Rocha, E.M.; Cunha, D.A.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A. Identification of Insulin in the Tear Film and Insulin Receptor and IGF-1 Receptor on the Human Ocular Surface. Investig. Ophthalmol. Vis. Sci. 2002, 43, 963–967.
- Robertson, D.M.; Zhu, M.; Wu, Y.-C. Cellular Distribution of the IGF-1R in Corneal Epithelial Cells. Exp. Eye Res. 2012, 94, 179–186.
- Titone, R.; Robertson, D.M. Insulin Receptor Preserves Mitochondrial Function by Binding VDAC1 in Insulin Insensitive Mucosal Epithelial Cells. FASEB J. 2020, 34, 754–775.
- Wu, Y.-C.; Zhu, M.; Robertson, D.M. Novel Nuclear Localization and Potential Function of Insulin-like Growth Factor-1 Receptor/Insulin Receptor Hybrid in Corneal Epithelial Cells. PLoS ONE 2012, 7, e42483.
- Jiang, Y.; Ju, Z.; Zhang, J.; Liu, X.; Tian, J.; Mu, G. Effects of Insulin-like Growth Factor 2 and Its Receptor Expressions on Corneal Repair. Int. J. Clin. Exp. Pathol. 2015, 8, 10185–10191.
- Bohnsack, R.N.; Warejcka, D.J.; Wang, L.; Gillespie, S.R.; Bernstein, A.M.; Twining, S.S.; Dahms, N.M. Expression of Insulin-like Growth Factor 2 Receptor in Corneal Keratocytes during Differentiation and in Response to Wound Healing. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7697–7708.
- Tests, D.; Diabetes, F.O.R. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2015, 38, S8–S16.
- Takahashi, H.; Ohara, K.; Ohmura, T.; Takahashi, R.; Zieske, J.D. Glucose Transporter 1 Expression in Corneal Wound Repair under High Serum Glucose Level. Jpn. J. Ophthalmol. 2000, 44, 470–474.
- Shanley, L.J.; McCaig, C.D.; Forrester, J.V.; Zhao, M. Insulin, Not Leptin, Promotes in Vitro Cell Migration to Heal Monolayer Wounds in Human Corneal Epithelium. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1088–1094.
- Lyu, J.; Lee, K.-S.; Joo, C.-K. Transactivation of EGFR Mediates Insulin-Stimulated ERK1/2 Activation and Enhanced Cell Migration in Human Corneal Epithelial Cells. Mol. Vis. 2006, 12, 1403–1410.
- Peterson, C.; Chandler, H.L. Insulin Facilitates Corneal Wound Healing in the Diabetic Environment through the RTK-PI3K/Akt/MTOR Axis in Vitro. Mol. Cell. Endocrinol. 2022, 548, 111611.
- Herencia-Bueno, K.E.; Aldrovani, M.; Crivelaro, R.M.; Thiesen, R.; Barros-Sobrinho, A.A.F.; Claros-Chacaltana, F.D.Y.; Padua, I.R.M.; Santos, D.M.; Laus, J.L. Reduction in Histone H3 Acetylation and Chromatin Remodeling in Corneas of Alloxan-Induced Diabetic Rats. Cornea 2018, 37, 624–632.
- Song, F.; Xue, Y.; Dong, D.; Liu, J.; Fu, T.; Xiao, C.; Wang, H.; Lin, C.; Liu, P.; Zhong, J.; et al. Insulin Restores an Altered Corneal Epithelium Circadian Rhythm in Mice with Streptozotocin-Induced Type 1 Diabetes. Sci. Rep. 2016, 6, 32871.
- de Cássia Alves, M.; Carvalheira, J.B.; Módulo, C.M.; Rocha, E.M. Tear Film and Ocular Surface Changes in Diabetes Mellitus. Arq. Bras. Oftalmol. 2008, 71, 96–103.
- Módulo, C.M.; Jorge, A.G.; Dias, A.C.; Braz, A.M.; Bertazolli-Filho, R.; Jordão, A.A.J.; Sérgio Marchini, J.; Rocha, E.M. Influence of Insulin Treatment on the Lacrimal Gland and Ocular Surface of Diabetic Rats. Endocrine 2009, 36, 161–168.
- Cunha, D.A.; de Alves, M.C.; Stoppiglia, L.F.; Jorge, A.G.; Módulo, C.M.; Carneiro, E.M.; Boschero, A.C.; Saad, M.J.A.; Velloso, L.A.; Rocha, E.M. Extra-Pancreatic Insulin Production in RAt Lachrymal Gland after Streptozotocin-Induced Islet Beta-Cells Destruction. Biochim. Biophys. Acta 2007, 1770, 1128–1135.
- Rocha, E.M.; Lima, M.H.d.M.; Carvalho, C.R.; Saad, M.J.; Velloso, L.A. Characterization of the Insulin-Signaling Pathway in Lacrimal and Salivary Glands of Rats. Curr. Eye Res. 2000, 21, 833–842.
- Di Zazzo, A.; Coassin, M.; Micera, A.; Mori, T.; De Piano, M.; Scartozzi, L.; Sgrulletta, R.; Bonini, S. Ocular Surface Diabetic Disease: A Neurogenic Condition? Ocul. Surf. 2021, 19, 218–223.
- Manchikanti, V.; Kasturi, N.; Rajappa, M.; Gochhait, D. Ocular Surface Disorder among Adult Patients with Type II Diabetes Mellitus and Its Correlation with Tear Film Markers: A Pilot Study. Taiwan J. Ophthalmol. 2021, 11, 156–160.
- Çakır, B.K.; Katırcıoğlu, Y.; Ünlü, N.; Duman, S.; Üstün, H. Ocular Surface Changes in Patients Treated with Oral Antidiabetic Drugs or Insulin. Eur. J. Ophthalmol. 2016, 26, 303–306.
- Purushothaman, I.; Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Ocular Surface Complications in Diabetes: The Interrelationship between Insulin and Enkephalin. Biochem. Pharmacol. 2021, 192, 114712.
- Quadrado, M.J.; Popper, M.; Morgado, A.M.; Murta, J.N.; Van Best, J.A. Diabetes and Corneal Cell Densities in Humans by in Vivo Confocal Microscopy. Cornea 2006, 25, 761–768.
- Taylor, H.R.; Kimsey, R.A. Corneal Epithelial Basement Membrane Changes in Diabetes. Investig. Ophthalmol. Vis. Sci. 1981, 20, 548–553.
- Qu, J.H.; Li, L.; Tian, L.; Zhang, X.Y.; Thomas, R.; Sun, X.G. Epithelial Changes with Corneal Punctate Epitheliopathy in Type 2 Diabetes Mellitus and Their Correlation with Time to Healing. BMC Ophthalmol. 2018, 18, 1.
- Kowalczuk, L.; Latour, G.; Bourges, J.-L.; Savoldelli, M.; Jeanny, J.-C.; Plamann, K.; Schanne-Klein, M.-C.; Behar-Cohen, F. Multimodal Highlighting of Structural Abnormalities in Diabetic Rat and Human Corneas. Transl. Vis. Sci. Technol. 2013, 2, 3.
- Ishibashi, F.; Kawasaki, A.; Yamanaka, E.; Kosaka, A.; Uetake, H. Morphometric Features of Corneal Epithelial Basal Cells, and Their Relationship with Corneal Nerve Pathology and Clinical Factors in Patients with Type 2 Diabetes. J. Diabetes Investig. 2013, 4, 492–501.
- Zagon, I.S.; Sassani, J.W.; Purushothaman, I.; McLaughlin, P.J. Blockade of OGFr Delays the Onset and Reduces the Severity of Diabetic Ocular Surface Complications. Exp. Biol. Med. 2021, 246, 629–636.
- Zagon, I.S.; Sassani, J.W.; Purushothaman, I.; McLaughlin, P.J. Dysregulation of the OGF–OGFr Pathway Correlates with Elevated Serum OGF and Ocular Surface Complications in the Diabetic Rat. Exp. Biol. Med. 2020, 245, 1414–1421.
- Purushothaman, I.; Zagon, I.S.; Sassani, J.W.; Zhou, S.; McLaughlin, P.J. Sex Differences in the Magnitude of Diabetic Ocular Surface Complications: Role of Serum OGF. Physiol. Behav. 2021, 237, 113436.
- Nureen, L.; Di Girolamo, N. Limbal Epithelial Stem Cells in the Diabetic Cornea. Cells 2023, 12, 2458.
- Chen, D.; Wang, L.; Guo, X.; Zhang, Z.; Xu, X.; Jin, Z.-B.; Liang, Q. Evaluation of Limbal Stem Cells in Patients With Type 2 Diabetes: An In Vivo Confocal Microscopy Study. Cornea 2024, 43, 67–75.
- Saghizadeh, M.; Soleymani, S.; Harounian, A.; Bhakta, B.; Troyanovsky, S.M.; Brunken, W.J.; Pellegrini, G.; Ljubimov, A.V. Alterations of Epithelial Stem Cell Marker Patterns in Human Diabetic Corneas and Effects of C-Met Gene Therapy. Mol. Vis. 2011, 17, 2177–2190.
- Kramerov, A.A.; Saghizadeh, M.; Ljubimov, A.V. Adenoviral Gene Therapy for Diabetic Keratopathy: Effects on Wound Healing and Stem Cell Marker Expression in Human Organ-Cultured Corneas and Limbal Epithelial Cells. J. Vis. Exp. 2016, 110, e54058.
- Ueno, H.; Hattori, T.; Kumagai, Y.; Suzuki, N.; Ueno, S.; Takagi, H. Alterations in the Corneal Nerve and Stem/Progenitor Cells in Diabetes: Preventive Effects of Insulin-like Growth Factor-1 Treatment. Int. J. Endocrinol. 2014, 2014, 312401.
- Zhang, Z.; Yang, L.; Li, Y.; Sun, D.; Chen, R.; Dou, S.; Liu, T.; Zhang, S.; Zhou, Q.; Xie, L. Interference of Sympathetic Overactivation Restores Limbal Stem/Progenitor Cells Function and Accelerates Corneal Epithelial Wound Healing in Diabetic Mice. Biomed. Pharmacother. 2023, 161, 114523.
- Kramerov, A.A.; Saghizadeh, M.; Maguen, E.; Rabinowitz, Y.S.; Ljubimov, A.V. Persistence of Reduced Expression of Putative Stem Cell Markers and Slow Wound Healing in Cultured Diabetic Limbal Epithelial Cells. Mol. Vis. 2015, 21, 1357–1367.
- Mort, R.L.; Douvaras, P.; Morley, S.D.; Dorà, N.; Hill, R.E.; Collinson, J.M.; West, J.D. Stem Cells and Corneal Epithelial Maintenance: Insights from the Mouse and Other Animal Models. Results Probl. Cell Differ. 2012, 55, 357–394.
- Ueno, H.; Ferrari, G.; Hattori, T.; Saban, D.R.; Katikireddy, K.R.; Chauhan, S.K.; Dana, R. Dependence of Corneal Stem/Progenitor Cells on Ocular Surface Innervation. Investig. Ophthalmol. Vis. Sci. 2012, 53, 867–872.
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Zirie, M.A.; Qazi, M.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; et al. Painful Diabetic Neuropathy Is Associated with Increased Nerve Regeneration in Patients with Type 2 Diabetes Undergoing Intensive Glycemic Control. J. Diabetes Investig. 2021, 12, 1642–1650.
- Mahelková, G.; Burdová, M.Č.; Malá, Š.; Hoskovcová, L.; Dotřelová, D.; Štechová, K. Higher Total Insulin Dose Has Positive Effect on Corneal Nerve Fibers in DM1 Patients. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3800–3807.
- Issar, T.; Tummanapalli, S.S.; Kwai, N.C.G.; Chiang, J.C.B.; Arnold, R.; Poynten, A.M.; Markoulli, M.; Krishnan, A. V Associations between Acute Glucose Control and Peripheral Nerve Structure and Function in Type 1 Diabetes. Diabet. Med. 2020, 37, 1553–1560.
- He, J.; Bazan, H.E.P. Mapping the Nerve Architecture of Diabetic Human Corneas. Ophthalmology 2012, 119, 956–964.
- Misra, S.L.; Slater, J.A.; McGhee, C.N.J.; Pradhan, M.; Braatvedt, G.D. Corneal Confocal Microscopy in Type 1 Diabetes Mellitus: A Six-Year Longitudinal Study. Transl. Vis. Sci. Technol. 2022, 11, 17.
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Holmes, A.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of Diet-Induced Obesity or Type 1 or Type 2 Diabetes on Corneal Nerves and Peripheral Neuropathy in C57Bl/6J Mice. J. Peripher. Nerv. Syst. 2015, 20, 24–31.
- Yorek, M.S.; Obrosov, A.; Shevalye, H.; Lupachyk, S.; Harper, M.M.; Kardon, R.H.; Yorek, M.A. Effect of Glycemic Control on Corneal Nerves and Peripheral Neuropathy in Streptozotocin-Induced Diabetic C57Bl/6J Mice. J. Peripher. Nerv. Syst. 2014, 19, 205–217.
- Davidson, E.P.; Coppey, L.J.; Kardon, R.H.; Yorek, M.A. Differences and Similarities in Development of Corneal Nerve Damage and Peripheral Neuropathy and in Diet-Induced Obesity and Type 2 Diabetic Rats. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1222–1230.
- Chen, D.K.; Frizzi, K.E.; Guernsey, L.S.; Ladt, K.; Mizisin, A.P.; Calcutt, N.A. Repeated Monitoring of Corneal Nerves by Confocal Microscopy as an Index of Peripheral Neuropathy in Type-1 Diabetic Rodents and the Effects of Topical Insulin. J. Peripher. Nerv. Syst. 2013, 18, 306–315.
- Chan, K.; Badanes, Z.; Ledbetter, E.C. Decreased Corneal Subbasal Nerve Fiber Length and Density in Diabetic Dogs with Cataracts Using in Vivo Confocal Microscopy. Vet. Ophthalmol. 2023, 26, 524–531.
- Machet, J.; Park, M.; Richardson, A.; Carnell, M.; Mouat, M.A.; Smith, N.J.; Turner, N.; Cochran, B.J.; Rye, K.A.; Di Girolamo, N. Type 2 Diabetes Influences Intraepithelial Corneal Nerve Parameters and Corneal Stromal-Epithelial Nerve Penetration Sites. J. Diabetes Investig. 2023, 14, 591–601.
- Markoulli, M.; Flanagan, J.; Tummanapalli, S.S.; Wu, J.; Willcox, M. The Impact of Diabetes on Corneal Nerve Morphology and Ocular Surface Integrity. Ocul. Surf. 2018, 16, 45–57.
- Zhou, T.; Lee, A.; Lo, A.C.Y.; Kwok, J.S.W.J. Diabetic Corneal Neuropathy: Pathogenic Mechanisms and Therapeutic Strategies. Front. Pharmacol. 2022, 13, 816062.
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130.
- Deardorff, P.M.; McKay, T.B.; Wang, S.; Ghezzi, C.E.; Cairns, D.M.; Abbott, R.D.; Funderburgh, J.L.; Kenyon, K.R.; Kaplan, D.L. Modeling Diabetic Corneal Neuropathy in a 3D In Vitro Cornea System. Sci. Rep. 2018, 8, 17294.
- Cui, H.; Liu, Y.; Qin, L.; Wang, L.; Huang, Y. Increased Membrane Localization of Pannexin1 in Human Corneal Synaptosomes Causes Enhanced Stimulated ATP Release in Chronic Diabetes Mellitus. Medicine 2016, 95, e5084.
- Yang, S.; Zhang, Y.; Zhang, Z.; Dan, J.; Zhou, Q.; Wang, X.; Li, W.; Zhou, L.; Yang, L.; Xie, L. Insulin Promotes Corneal Nerve Repair and Wound Healing in Type 1 Diabetic Mice by Enhancing Wnt/β-Catenin Signaling. Am. J. Pathol. 2020, 190, 2237–2250.
- Ponirakis, G.; Abdul-Ghani, M.A.; Jayyousi, A.; Zirie, M.A.; Al-Mohannadi, S.; Almuhannadi, H.; Petropoulos, I.N.; Khan, A.; Gad, H.; Migahid, O.; et al. Insulin Resistance Limits Corneal Nerve Regeneration in Patients with Type 2 Diabetes Undergoing Intensive Glycemic Control. J. Diabetes Investig. 2021, 12, 2002–2009.
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531.
- Diener, C.; Keller, A.; Meese, E. Emerging Concepts of MiRNA Therapeutics: From Cells to Clinic. Trends Genet. 2022, 38, 613–626.
- Park, J.K.; Peng, H.; Katsnelson, J.; Yang, W.; Kaplan, N.; Dong, Y.; Rappoport, J.Z.; He, C.; Lavker, R.M. MicroRNAs-103/107 Coordinately Regulate Macropinocytosis and Autophagy. J. Cell Biol. 2016, 215, 667–685.
- Wang, L.; Xu, X.; Chen, Q.; Wei, Y.; Wei, Z.; Jin, Z.-B.; Liang, Q. Extracellular Vesicle MicroRNAs From Corneal Stromal Stem Cell Enhance Stemness of Limbal Epithelial Stem Cells by Targeting the Notch Pathway. Investig. Ophthalmol. Vis. Sci. 2023, 64, 42.
- Funari, V.A.; Winkler, M.; Brown, J.; Dimitrijevich, S.D.; Ljubimov, A.V.; Saghizadeh, M. Differentially Expressed Wound Healing-Related MicroRNAs in the Human Diabetic Cornea. PLoS ONE 2013, 8, e84425.
- Winkler, M.A.; Dib, C.; Ljubimov, A.V.; Saghizadeh, M. Targeting MiR-146a to Treat Delayed Wound Healing in Human Diabetic Organ-Cultured Corneas. PLoS ONE 2014, 9, e114692.
- Poe, A.J.; Shah, R.; Khare, D.; Kulkarni, M.; Phan, H.; Ghiam, S.; Punj, V.; Ljubimov, A.V.; Saghizadeh, M. Regulatory Role of MiR-146a in Corneal Epithelial Wound Healing via Its Inflammatory Targets in Human Diabetic Cornea: MiR-146a Inflammatory Role in Diabetic Corneal Epithelial Wound Healing. Ocul. Surf. 2022, 25, 92–100.
- Kulkarni, M.; Leszczynska, A.; Wei, G.; Winkler, M.A.; Tang, J.; Funari, V.A.; Deng, N.; Liu, Z.; Punj, V.; Deng, S.X.; et al. Genome-Wide Analysis Suggests a Differential MicroRNA Signature Associated with Normal and Diabetic Human Corneal Limbus. Sci. Rep. 2017, 7, 3448.
- Leszczynska, A.; Kulkarni, M.; Ljubimov, A.V.; Saghizadeh, M. Exosomes from Normal and Diabetic Human Corneolimbal Keratocytes Differentially Regulate Migration, Proliferation and Marker Expression of Limbal Epithelial Cells. Sci. Rep. 2018, 8, 15173.
- Klocek, M.S.; Sassani, J.W.; McLaughlin, P.J.; Zagon, I.S. Naltrexone and Insulin Are Independently Effective but Not Additive in Accelerating Corneal Epithelial Healing in Type I Diabetic Rats. Exp. Eye Res. 2009, 89, 686–692.
- Zagon, I.S.; Sassani, J.W.; McLaughlin, P.J. Insulin Treatment Ameliorates Impaired Corneal Reepithelialization in Diabetic Rats. Diabetes 2006, 55, 1141–1147.
- Aynsley, T.R. The use of insulin in the treatment of corneal ulcers. Br. J. Ophthalmol. 1945, 29, 361–363.
- Fai, S.; Ahem, A.; Mustapha, M.; Mohd Noh, U.K.; Bastion, M.-L.C. Randomized Controlled Trial of Topical Insulin for Healing Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Asia-Pac. J. Ophthalmol. 2017, 6, 418–424.
- Dasrilsyah, A.M.; Wan Abdul Halim, W.H.; Mustapha, M.; Tang, S.F.; Kaur, B.; Ong, E.Y.; Catherine Bastion, M.L. Randomized Clinical Trial of Topical Insulin Versus Artificial Tears for Healing Rates of Iatrogenic Corneal Epithelial Defects Induced During Vitreoretinal Surgery in Diabetics. Cornea 2023, 42, 1395–1403.
- Balal, S.; Din, N.; Ashton, C.; Ahmad, S. Healing of Chemical Injury-Related Persistent Corneal Epithelial Defects With Topical Insulin. Cornea 2022, 42, 1000–1004.
- Diaz-Valle, D.; Burgos-Blasco, B.; Gegundez-Fernandez, J.A.; Garcia-Caride, S.; Puebla-Garcia, V.; Peña-Urbina, P.; Benitez-del-Castillo, J.M. Topical Insulin for Refractory Persistent Corneal Epithelial Defects. Eur. J. Ophthalmol. 2021, 31, 2280–2286.
- Esmail, A.; Ibrahim, M.; Nage, S. Efficacy of Topical Insulin for Recurrent Epithelial Corneal Erosions. Ir. J. Med. Sci. 2023, 192, 3117–3123.
- Soares, R.J.D.S.M.; Arêde, C.; Sousa Neves, F.; da Silva Fernandes, J.; Cunha Ferreira, C.; Sequeira, J. Topical Insulin-Utility and Results in Refractory Neurotrophic Keratopathy in Stages 2 and 3. Cornea 2022, 41, 990–994.
- Diaz-Valle, D.; Burgos-Blasco, B.; Rego-Lorca, D.; Puebla-Garcia, V.; Perez-Garcia, P.; Benitez-Del-Castillo, J.M.; Herrero-Vanrell, R.; Vicario-de-la-Torre, M.; Gegundez-Fernandez, J.A. Comparison of the Efficacy of Topical Insulin with Autologous Serum Eye Drops in Persistent Epithelial Defects of the Cornea. Acta Ophthalmol. 2022, 100, e912–e919.
- Wang, A.L.; Weinlander, E.; Metcalf, B.M.; Barney, N.P.; Gamm, D.M.; Nehls, S.M.; Struck, M.C. Use of Topical Insulin to Treat Refractory Neurotrophic Corneal Ulcers. Cornea 2017, 36, 1426–1428.
- Bastion, M.L.C.; Ling, K.P. Topical Insulin for Healing of Diabetic Epithelial Defects?: A Retrospective Review of Corneal Debridement during Vitreoretinal Surgery in Malaysian Patients. Med. J. Malays. 2013, 68, 208–216.
- Burgos-Blasco, B.; Diaz-Valle, D.; Rego-Lorca, D.; Perez-Garcia, P.; Puebla-Garcia, V.; Fernandez-Vigo, J.I.; Benitez-Del-Castillo, J.M.; Gegundez-Fernandez, J.A. Topical Insulin, a Novel Corneal Epithelial Regeneration Agent in Dry Eye Disease. Eur. J. Ophthalmol. 2023, 11206721231206790.
- Serrano-Giménez, R.; Contreras-Macías, E.; García-Bernal, A.; Fobelo-Lozano, M.J. Insulina Tópica En El Tratamiento de Úlcera Corneal Refractaria En Un Paciente No Diabético: A Propósito de Un Caso. Farm. Hosp. 2020, 44, 297–299.
- Tong, C.M.; Iovieno, A.; Yeung, S.N. Topical Insulin for Neurotrophic Corneal Ulcers. Can. J. Ophthalmol. 2020, 55, e170–e172.
- Galvis, V.; Niño, C.A.; Tello, A.; Grice, J.M.; Gómez, M.A. Topical Insulin in Neurotrophic Keratopathy after Resection of Acoustic Neuroma. Arch. Soc. Esp. Oftalmol. 2019, 94, 100–104.
- Giannaccare, G.; Coco, G.; Rossi, C.; Borselli, M.; Lucisano, A.; Vaccaro, S.; Verdiglione, M.; Scorcia, V. Combined Use of Therapeutic Hyper-CL Soft Contact Lens and Insulin Eye Drops for the Treatment of Recalcitrant Neurotrophic Keratopathy. Cornea 2023, 43, 120–124.
- Khilji, M.; Tanveer, S.; Khan, F.Z.; Yazdan, D.A.; Khilji, A. Neurotrophic Keratopathy and Topical Insulin Therapy: A Case Report. Cureus 2023, 15, 7–11.
- Anitua, E.; de la Fuente, M.; Sánchez-Ávila, R.M.; de la Sen-Corcuera, B.; Merayo-Lloves, J.; Muruzábal, F. Beneficial Effects of Plasma Rich in Growth Factors (PRGF) Versus Autologous Serum and Topical Insulin in Ocular Surface Cells. Curr. Eye Res. 2023, 48, 456–464.
- Castro Mora, M.P.; Palacio Varona, J.; Perez Riaño, B.; Laverde Cubides, C.; Rey-Rodriguez, D.V. Effectiveness of Topical Insulin for the Treatment of Surface Corneal Pathologies. Arch. La Soc. Española Oftalmol. 2023, 98, 220–232.
- Jaworski, M.; Lorenc, A.; Leszczyński, R.; Mrukwa-Kominek, E. Topical Insulin in Neurotrophic Keratopathy: A Review of Current Understanding of the Mechanism of Action and Therapeutic Approach. Pharmaceutics 2023, 16, 15.
- Cruz-Cazarim, E.L.C.; Cazarim, M.S.; Ogunjimi, A.T.; Petrilli, R.; Rocha, E.M.; Lopez, R.F. V Prospective Insulin-Based Ophthalmic Delivery Systems for the Treatment of Dry Eye Syndrome and Corneal Injuries. Eur. J. Pharm. Biopharm. 2019, 140, 1–10.
- Voronova, A.; Prieto, C.; Pardo-Figuerez, M.; Lagaron, J.M.; Sanyal, A.; Demir, B.; Hubert, T.; Plaisance, V.; Pawlowski, V.; Vignoud-Despond, S.; et al. Photothermal Activatable Mucoadhesive Fiber Mats for On-Demand Delivery of Insulin via Buccal and Corneal Mucosa. ACS Appl. Bio. Mater. 2022, 5, 771–778.
- Liao, Y.; Jiang, H.; Du, Y.; Xiong, X.; Zhang, Y.; Du, Z. Using Convolutional Neural Network as a Statistical Algorithm to Explore the Therapeutic Effect of Insulin Liposomes on Corneal Inflammation. Comput. Intell. Neurosci. 2022, 2022, 1169438.
- Xiong, X.; Jiang, H.; Liao, Y.; Du, Y.; Zhang, Y.; Wang, Z.; Zheng, M.; Du, Z. Liposome–Trimethyl Chitosan Nanoparticles Codeliver Insulin and SiVEGF to Treat Corneal Alkali Burns by Inhibiting Ferroptosis. Bioeng. Transl. Med. 2023, 8, e10499.




