Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Boris Chauviré | -- | 1251 | 2024-03-01 13:35:28 | | | |
| 2 | Catherine Yang | + 1 word(s) | 1252 | 2024-03-04 02:05:33 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Chauviré, B.; Mollé, V.; Guichard, F.; Rondeau, B.; Thomas, P.S.; Fritsch, E. Cracking of Gem Opals. Encyclopedia. Available online: https://encyclopedia.pub/entry/55770 (accessed on 07 February 2026).
Chauviré B, Mollé V, Guichard F, Rondeau B, Thomas PS, Fritsch E. Cracking of Gem Opals. Encyclopedia. Available at: https://encyclopedia.pub/entry/55770. Accessed February 07, 2026.
Chauviré, Boris, Valentin Mollé, Florine Guichard, Benjamin Rondeau, Paul Stephen Thomas, Emmanuel Fritsch. "Cracking of Gem Opals" Encyclopedia, https://encyclopedia.pub/entry/55770 (accessed February 07, 2026).
Chauviré, B., Mollé, V., Guichard, F., Rondeau, B., Thomas, P.S., & Fritsch, E. (2024, March 01). Cracking of Gem Opals. In Encyclopedia. https://encyclopedia.pub/entry/55770
Chauviré, Boris, et al. "Cracking of Gem Opals." Encyclopedia. Web. 01 March, 2024.
Copy Citation
The value of gem opals is compromised by their potential susceptibility to “crazing”, a phenomenon observed either in the form of whitening or cracking.
opal
cracking
water
TGA
drying
Gems
1. Introduction
Opals are well-known gems offering a wide range of aspects and optical phenomena, particularly light diffraction in “noble” opals. The very best specimens reach very high prices in the market. However, the reputation of gem opals is tarnished by its potential to destabilize (or “craze”) in two different ways: It may lose its transparency (“whitening”) or develop fissures (cracking) either at the surface or in its center (examples of cracked opals are shown in Figure 1) [1]. This, of course, alters considerably the value of gem opals. Both phenomena have already been described, but they are not fully understood. Early studies have shown that “whitening“ is correlated with an increase in porosity and a reorganization of the hydration states without changing the silica framework [2][3]. The first empirical investigations on cracking suggest that the drying of opals is a key factor for understanding cracking [4][5][6]. Nevertheless, there remains a need for an experimental or theoretical framework to recognize ahead of time that opals may “craze”. This research concentrates on the understanding of crazing due to cracking. In order to address the issue of instability in opals and its detection, the materials and their structure and properties must first be understood. Therefore, the researchers first review current knowledge on opal structure, water content, and stability and then experimentally crack a variety of opals to assess the physical origin of this degradation process.
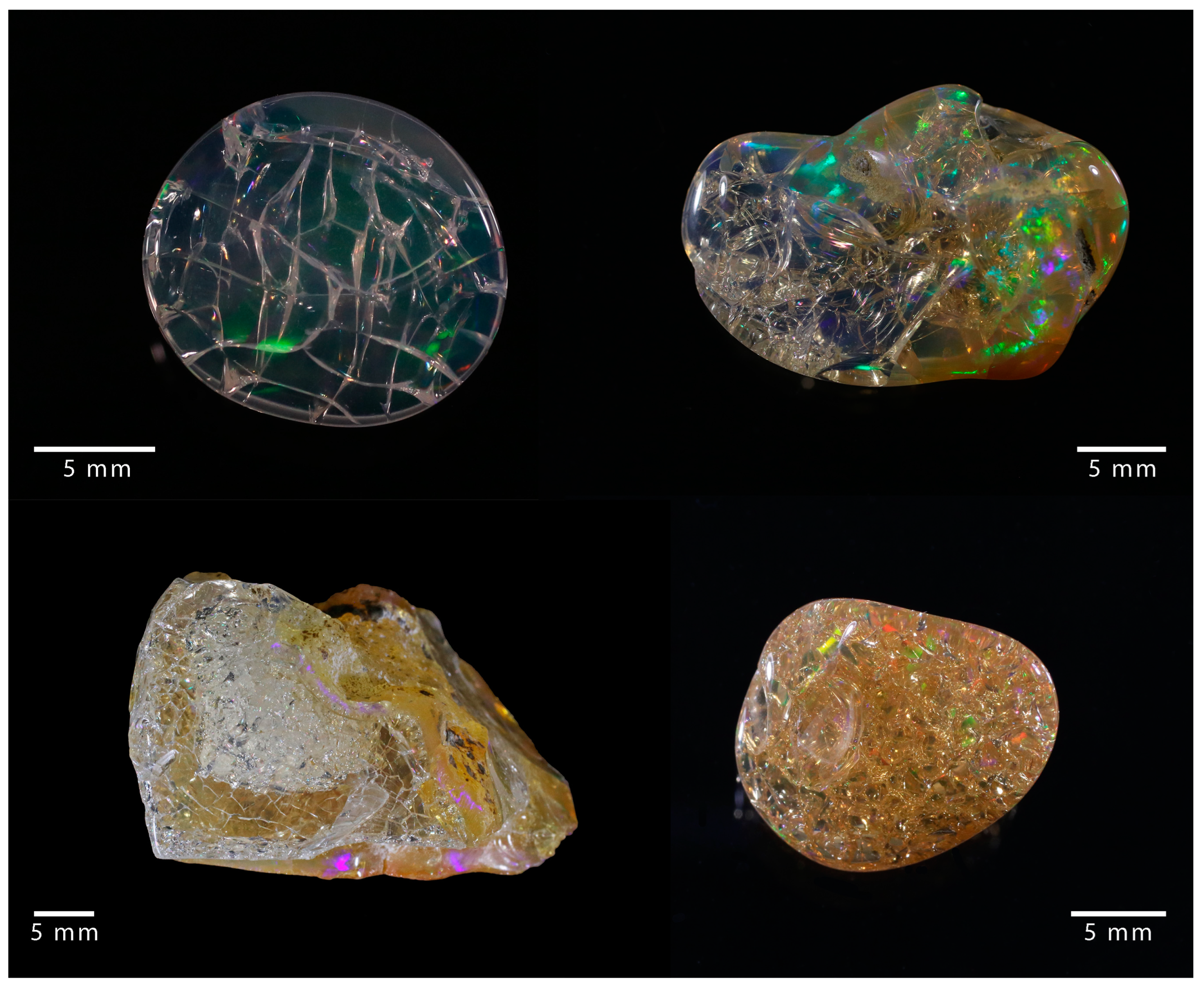
Figure 1. Examples of cracked opal, where a network of fractures developed.
2. Opal
Opal refers to natural hydrated varieties of non- to nanocrystalline silica (SiO2·nH2O) frequently found in a wide range of geological contexts where the aqueous alteration of silicate minerals occurs. A crystallographic classification based on X-ray diffraction (XRD) distinguishes amorphous opal (opal-A) from paracrystalline opal. The latter is further subdivided into opal-CT, where broad cristobalite and tridymite diffraction peaks are observed, and opal-C, where only cristobalite peaks are visible [7]. It has been suggested that opal-CT is a poorly crystallized cristobalite with tridymite stacking [7], but recent structural studies indicated that opal-CT may comprise the nano-domains of diffracting tridymites alternating with non-diffracting silica, with no evidence of cristobalite domains [8][9][10][11]. Opal-A, displaying a broad band typical of amorphous material in XRD, can be subdivided into two sub-types [12]:
The distinction between opal types, even if firstly defined by X-ray diffraction, can also be achieved by Raman spectroscopy [22][23][24], infrared spectroscopy (either in the mid-infrared sensitive to silica framework vibrations [24][25][26] or in the near-infrared sensitive to the hydration state [12][27][28]), nuclear magnetic resonance [29][30], and by observing microstructural features by electron microscopy [9][18][31].
Both opals types are found in various geological contexts. Opal-A is a common phase constituting the silica deposits around hot springs [32][33][34] or during the weathering of rocks [35][36], with a formation temperature ranging from room temperature up to 100 °C and probably more for opal-AN. Similarly, opal-CT can form in a hydrothermal context such as Mexican deposits [37] or by the weathering of volcanic rocks as observed in the Wollo province in Ethiopia [38][39][40] with a formation temperature ranging from ambient up to 160 °C.
Microstructural features observed in opals are all based on nanograins of ca. 25 nm for opal-A [18][41] and of 10 to 50 nm for opal-CT (with an average near 25 nm, including tridymite nano-domains) [18][42][43]. In opal-A, nanograins usually aggregate in spheres ranging from 80 nm to 8 µm in diameter. Opal-CT displays a greater variety of microstructural features where nanograins may accumulate randomly in fibers, platelets, or lepispheres [18][42]. On rare occasions, monodispersed hundred-nanometer-sized silica spheres for opal-A or lepispheres for opal-CT may be arranged into ordered arrays enabling the diffraction of visible light, producing the play-of-colour (POC, patches of moving pure spectral colors) that is highly prized in precious or noble opals [21][44].
3. Water in Opals
In opals, water is present as molecular water (H2O) and chemically bound water in the form of silanol groups (Si-OH) [12][27][45][46][47]. Near-infrared spectroscopy indicates that much of an opal’s hydration consists of molecular water [12][27][48]. Early studies suggested that hydration is concentrated in the interstices between primary spheres [19], but further developments have identified more states in which water is present: interstitial (voids between primary spheres), pore, adsorbed at the surface, and as molecular water trapped in silica cages [12][49][50]. Similarly, silanol groups appear to be located at silica interfaces (such as defects such as broken Si-O-Si bridges in the bulk silica) [12].
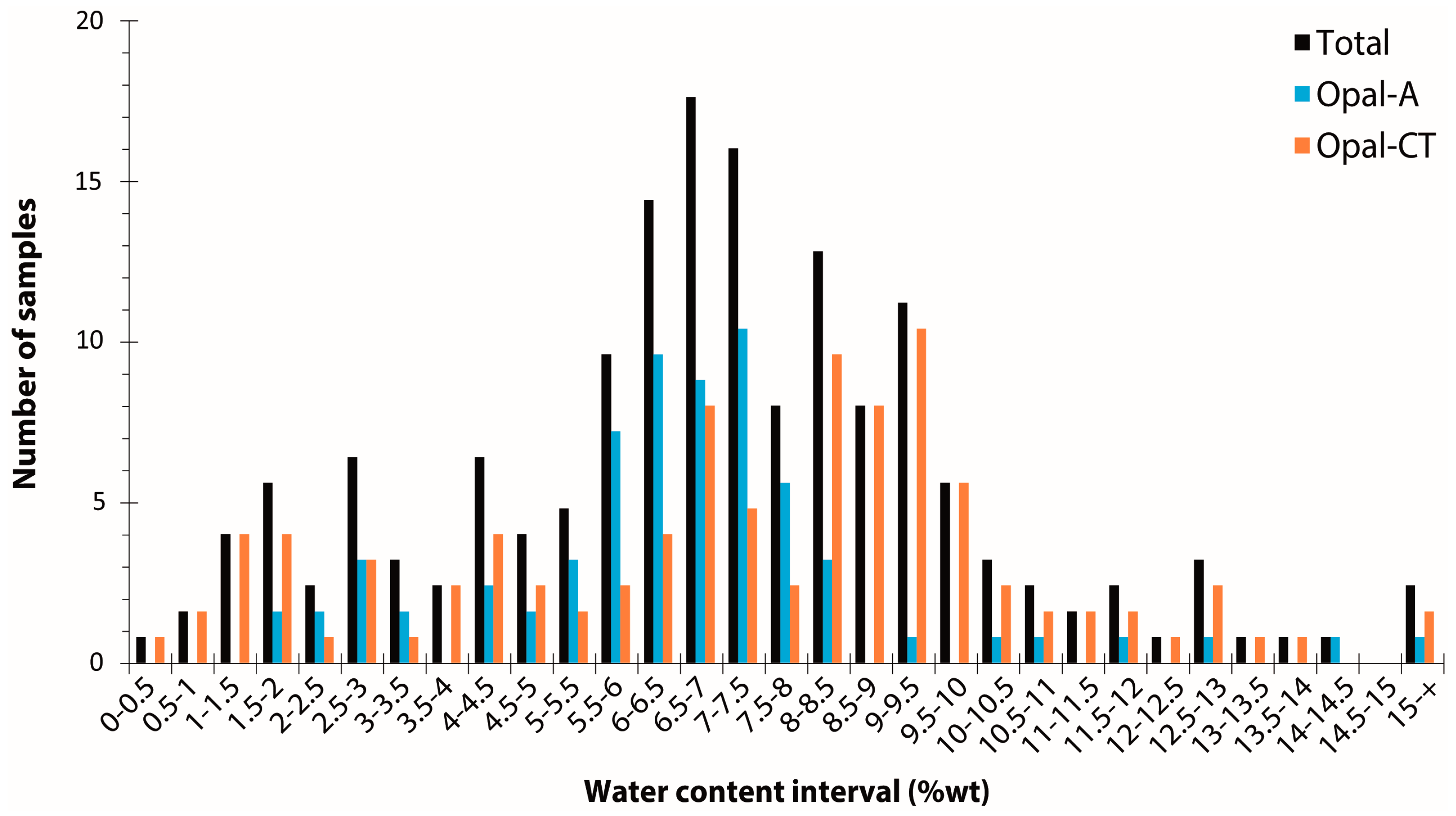
By collating 204 measurements (mostly by thermogravimetric analysis) from the literature [3][6][12][46][51][52][53][54][55][56][57][58][59][60][61], opal water content ranges from 0.5 to 18.1 weight percent, with an average at 6.99 wt% and a standard deviation of 3.05 (Figure 2). A very slight difference between opal-A and opal-CT (6.56 ± 2.41 wt% and 7.32 ± 3.43 wt%, respectively) is shown here between 82 opal-A and 118 opal-CT samples (four opals are not identified). Considering the average and standard deviation, statistically, opal-A and opal-CT are hardly distinguishable based on water content alone. Both opal types have a significant distribution in water content, especially at the lower water content end of the distribution. The opal types do, however, differ in terms of the shape of their distributions: Opal-A shows a distribution centred around 7 wt%, whereas opal-CT has two populations, with one close to opal-A and a second around 9 wt% (Figure 2). In addition, the difference between opals from various origins is statistically indistinguishable: Australia (70 data; 7.31 ± 2.11 wt%), Ethiopia (23 data; 8.43 ± 3.63 wt%), and Mexico (37 data; 8.32 ± 3.54 wt%).
Differential scanning calorimetry (DSC) has demonstrated that between 10 and 33% of the total water present is crystallisable [50][58]. From the melt temperature depression in DSC measurements, it is possible to estimate the pore size in which the crystallisable water is contained. It is estimated to be in the nanometer range (circa 4 to 10 nm in diameter for opal-CT and 6 to 200 (+) nm for opal-A) [50][58]. The crystallisable water contained in opal is present in both isolated and interconnected closed pores or, in some cases, in open pores exposed to the atmosphere, as demonstrated by measurements of the opal’s near-infrared signature at low pressures [62].
4. Instability of Opals by Cracking
In gemmology, instability is defined as an alteration of the aspect hindering the use of the stone for jewellery. Cracking is the development of cracks (fractures) in an initially homogeneous volume, and it obviously affects the integrity of the stone (Figure 3). In an attempt to understand cracking, early studies have attempted to induce cracking empirically, especially by sacrificing stones, although these studies provided no evidence that other samples from the same location would react similarly [4][5][6]. These first investigations revealed that drying rates have a significant impact on the initiation of cracking, suggesting that drying shrinkage in opals could be the main driver for cracking [5][6]. Cracks have been reported to occur primarily in transparent opals, specifically at the surface of the stone [1]. Some attempts have been made to prevent cracking in opals, particularly by applying a specific treatment after mining [63]. Despite a better knowledge of this process, no scientific nor objective criterion has been established at this point to assess or predict the stability of a given sample.

Figure 3. Example of an opal sample collected in 2013 at the mine site (Kok Woha, Wollo Province, Ethiopia, see [39] for details on mines) and the result after 3 years of storage in the atmosphere, where it was allowed to dry.
References
- Rondeau, B.; Fritsch, E.; Mazzero, F.; Gauthier, J. Opal – The Craze for Stability. Color 2011, 4, 2–5.
- Paris, M.; Fritsch, E.; Aguilar-Reyes, B. 1H, 29Si and 27Al NMR study of the destabilization process of a paracrystalline opal from Mexico. J. Non. Cryst. Solids 2007, 353, 1650–1656.
- Aguilar-Reyes, B. Etude microstructurale des opales: Application à la déstabilisation par blanchissement. Ph.D. Thesis, Université de Nantes, Nantes, France, 2004.
- Johnson, M.L.; Kammerling, R.C.; Deghionno, D.G.; Koivula, J.I. Opal from Shewa province, Ethiopia. Gems Gemol. 1996, 32, 112–120.
- Smith, K.L. Opals from Opal Butte, Oregon. Gems Gemol. 1988, 24, 229–236.
- Pearson, G. Role of Water in Cracking of Opal. Aust. Gemol. 1985, 15, 435–445.
- Jones, J.B.; Segnit, E.R. Nature of Opal Part I: Nomenclature and constituent phases. J. Geol. Soc. Aust. 1971, 18, 57–68.
- Fröhlich, F. The opal-CT nanostructure. J. Non. Cryst. Solids 2020, 533, 119938.
- Elzea, J.M.; Rice, S.B. Tem and X-Ray Diffraction Evidence for Cristobalite and Tridymite Stacking Sequences in Opal. Clays Clay Miner. 1996, 44, 492–500.
- Rice, S.B.; Freund, H.; Huang, W.-L.; Clouse, J.A.; Isaacs, C.M. Application of Fourier Transform Infrared Spectroscopy to Silica Diagenesis: The Opal-A to Opal-CT Transformation. SEPM J. Sediment. Res. 1995, 65A, 639–647.
- Wilson, M.J.; Russell, J.D.; Tait, J.M. A new interpretation of the structure of disordered α-cristobalite. Contrib. to Mineral. Petrol. 1974, 47, 1–6.
- Langer, K.; Flörke, O.W.W. Near infrared absorption spectra (4000-9000 cm−1) of opals and the role of “water” in these SiO2-nH2O minerals. Fortschritte der Mineral. 1974, 52, 17–51.
- Göttlicher, J.; Pentinghaus, H.J.; Himmel, B. On the Microstructure of Geyserites and Hyalites, Natural Hydrous Forms of Silica. J. Sol-Gel Sci. Technol. 1998, 13, 85–88.
- Flörke, O.W. Transport and Deposition of SiO2 with H2O under Supercritical Conditions. Krist. Und Tech. 1972, 7, 159–166.
- Plumeré, N.; Ruff, A.; Speiser, B.; Feldmann, V.; Mayer, H.A. Stöber silica particles as basis for redox modifications: Particle shape, size, polydispersity, and porosity. J. Colloid Interface Sci. 2012, 368, 208–219.
- Meier, M.; Ungerer, J.; Klinge, M.; Nirschl, H. Synthesis of nanometric silica particles via a modified Stöber synthesis route. Colloids Surfaces A Physicochem. Eng. Asp. 2018, 538, 559–564.
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interface Sci. 1968, 26, 62–69.
- Gaillou, E.; Fritsch, E.; Aguilar-Reyes, B.; Rondeau, B.; Post, J.; Barreau, A.; Ostroumov, M. Common gem opal: An investigation of micro- to nano-structure. Am. Mineral. 2008, 93, 1865–1873.
- Darragh, P.J.; Gaskin, A.J.; Terrell, B.C.; Sanders, J.V. Origin of Precious Opal. Nature 1966, 209, 13–16.
- Darragh, J.; Perdrix, J.L. Precious opal-developments towards synthesis. Aust. Gemol. 1973, 11, 17–21.
- Jones, J.B.; Sanders, J.V.; Segnit, E.R. Structure of Opal. Nature 1964, 204, 990–991.
- Smallwood, A.G. A preliminary investigation of precious opal by laser Raman spectroscopy. Aust. Gemol. 2000, 20, 363–366.
- Ostrooumov, M.; Fritsch, E.; Lasnier, B.; Lefrant, S. Spectres Raman des opales: Aspect diagnostique et aide à la classification. Eur. J. Mineral. 1999, 11, 899–908.
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. A Review of the Classification of Opal with Reference to Recent New Localities. Minerals 2019, 9, 299.
- Adamo, I.; Ghisoli, C.; Caucia, F. A contribution to the study of FTIR spectra of opals. Neues Jahrb. Mineral. Abhandlungen 2010, 187, 63–68.
- Wilson, M.J. The structure of opal-CT revisited. J. Non. Cryst. Solids 2014, 405, 68–75.
- Chauviré, B.; Rondeau, B.; Mangold, N. Near infrared signature of opal and chalcedony as a proxy for their structure and formation conditions. Eur. J. Mineral. 2017, 29, 409–421.
- Sun, V.Z.; Milliken, R.E. Distinct Geologic Settings of Opal-A and More Crystalline Hydrated Silica on Mars. Geophys. Res. Lett. 2018, 45, 10–221.
- Brown, L.D.; Ray, A.S.; Thomas, P.S. 29Si and 27Al NMR study of amorphous and paracrystalline opals from Australia. J. Non. Cryst. Solids 2003, 332, 242–248.
- Curtis, N.J.; Gascooke, J.R.; Johnston, M.R.; Pring, A. 29 Si Solid-State NMR Analysis of Opal-AG, Opal-AN and Opal-CT: Single Pulse Spectroscopy and Spin-Lattice T1 Relaxometry. Minerals 2022, 12, 323.
- Herdianita, N.R.; Browne, P.R.L.; Rodgers, K.A.; Campbell, K.A. Mineralogical and textural changes accompanying ageing of silica sinter. Miner. Depos. 2000, 35, 48–62.
- Jones, B.; Renaut, R.W. Formation of silica oncoids around geysers and hot springs at El Tatio, northern Chile. Sedimentology 1997, 44, 287–304.
- Vysotsky, S.V.; Barkar, A.V.; Kuryavy, V.G.; Chusovitin, E.A.; Karabtsov, A.A.; Safronov, P.P. Hydrothermal noble opal: Structure and genesis. Geol. Ore Depos. 2010, 52, 815–820.
- Vysotskiy, S.V.; Ignatiev, a.V.; Khlestunova, A.G.; Velivetskaya, T.A.; Okrugin, A.S. Peculiarities of the oxygen isotope ratio in precious opals. Russ. J. Pacific Geol. 2013, 7, 427–430.
- Rey, P.F. Opalisation of the Great Artesian Basin (central Australia): An Australian story with a Martian twist. Aust. J. Earth Sci. 2013, 60, 291–314.
- Townsend, I.J. The geology of Australian opal deposits. Aust. Gemol. 2001, 21, 34–37.
- Spencer, R.J.; Levinson, A.A.; Koivula, J.I. Opal from Quérétaro, Mexico: Fluid inclusion study. Gems Gemol. 1992, 28, 28.
- Rondeau, B.; Cenki-Tok, B.B.B.; Fritsch, E.; Mazzero, F.; Gauthier, J.-P.; Bodeur, Y.; Bekele, E.; Gaillou, E.; Ayalew, D. Geochemical and petrological characterization of gem opals from Wegel Tena, Wollo, Ethiopia: Opal formation in an Oligocene soil. Geochemistry Explor. Environ. Anal. 2012, 12, 93–104.
- Chauviré, B.; Rondeau, B.; Mazzero, F.; Ayalew, D. The Precious Opal Deposit At Wegel Tena, Ethiopia: Formation Via Successive Pedogenesis Events. Can. Mineral. 2017, 55, 701–723.
- Chauviré, B.; Rondeau, B.; Alexandre, A.; Chamard-Bois, S.; La, C.; Mazzero, F. Pedogenic origin of precious opals from Wegel Tena (Ethiopia): Evidence from trace elements and oxygen isotopes. Appl. Geochemistry 2019, 101, 127–139.
- Darragh, B.P.J.; Gaskin, A.J. The nature and origin of opal. Aust. Gemol. 1966, 8, 5–9.
- Fritsch, E.; Ostrooumov, M.; Rondeau, B.; Barreau, A.; Albertini, D.; Marie, A.-M.; Lasnier, B.; Wery, J. Mexican Gem Opals: Nano- and micro-structure, origin of colour, comparison with other common opals of gemological significance. Aust. Gemol. 2002, 21, 230–233.
- Fritsch, E.; Gaillou, E.; Rondeau, B.; Barreau, A.; Albertini, D.; Ostroumov, M. The nanostructure of fire opal. J. Non. Cryst. Solids 2006, 352, 3957–3960.
- Darragh, P.J.; Sanders, J. V The origin of colour in opal. Aust. Gemol. 1965, 7, 9–12.
- Graetsch, H.; Flörke, O.W.; Miehe, G. The nature of water in chalcedony and opal-C from brazilian agate geodes. Phys. Chem. Miner. 1985, 12, 300–306.
- Boboň, M.; Christy, A.A.; Kluvanec, D.; Illášová, L. State of water molecules and silanol groups in opal minerals: A near infrared spectroscopic study of opals from Slovakia. Phys. Chem. Miner. 2011, 38, 809–818.
- Smallwood, A.G.; Thomas, P.S.; Ray, A.S. Characterisation of the dehydration of australian sedimentary and volcanic precious opal by thermal methods. J. Therm. Anal. Calorim. 2008, 92, 91–95.
- Brown, L.D.; Ray, A.S.; Thomas, P.S.; Guerbois, J.P. Thermal characteristics of Australian sedimentary opals. J. Therm. Anal. Calorim. 2002, 68, 31–36.
- Smallwood, A.G.; Thomas, P.S.; Ray, A.S. The thermophysical properties of australian opal. In Proceedings of the 9th International Congress for Applied Mineralogy, Bisbane, Queensland, Australia, 8–10 September 2008; pp. 557–565.
- Thomas, P.S.; Guerbois, J.-P.; Smallwood, A.G. Low temperature DSC characterisation of water in opal. J. Therm. Anal. Calorim. 2013, 113, 1255–1260.
- Thomas, P.S.; Šesták, J.; Heide, K.; Fueglein, E.; Šimon, P. Thermal properties of Australian sedimentary opals and Czech moldavites. J. Therm. Anal. Calorim. 2010, 99, 861–867.
- Thomas, P.; Chauviré, B.; Flower-Donaldson, K.; Aldridge, L.; Smallwood, A.; Liu, B. FT-NIR and DSC characterisation of water in opal. Ceram. Int. 2020, 46, 29443–29450.
- Graetsch, H. Structural characteristics of opaline and microcrystalline silica minerals. Rev. Mineral. 1994, 29, 209–232.
- Gaillou, E. Relations entre nanostructure, propriétés physiques et mode de formation des opales A et CT. Ph.D. Thesis, Université de Nantes, Nantes, France, 2016.
- Thomas, P.S.; Aldridge, L.; Smallwood, A. Water in opal - what can it tell us ? Color 2019, 41, 63–69.
- Adams, S.J.; Hawkes, G.E.; Curzon, E.H. A solid state 29Si nuclear magnetic resonance study of opal and other hydrous silicas. Am. Mineral. 1991, 76, 1863–1871.
- Bayliss, P.; Males, P.A. The Mineralogical Similarity of Precious and Common Opal from Australia. Mineral. Mag. 1965, 35, 429–431.
- Chauviré, B.; Thomas, P.S. DSC of natural opal: Insights into the incorporation of crystallisable water in the opal microstructure. J. Therm. Anal. Calorim. 2020, 140, 2077–2085.
- De Jong, B.H.W.S.; Van Hoek, J.; Veeman, W.S.; Manson, D. V X-ray diffraction and 29Si magic-angle-spinning NMR of opals; incoherent long-and short-range order in opal-CT. Am. Mineral. 1987, 72, 1195–1203.
- Gallacher, A.D. Geochemistry of Sedimentary Opal, Hebel, Southern Queensland; University of Melbourne: Melbourne, Australia, 2001.
- Segnit, E.R.; Stevens, T.J.; Jones, J.B. The role of water in opal. J. Geol. Soc. Aust. 1965, 12, 211–226.
- Chauviré, B.; Pineau, M.; Quirico, E.; Beck, P. Near infrared signature of opaline silica at Mars-relevant pressure and temperature. Earth Planet. Sci. Lett. 2021, 576, 117239.
- Filin, S.V.; Puzynin, A.I. Prevention of cracking in Ethiopian opal. Aust. Gemmol. 2009, 23, 579–582.
More
Information
Subjects:
Mineralogy
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
873
Revisions:
2 times
(View History)
Update Date:
04 Mar 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




