Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Mineralogy
The value of gem opals is compromised by their potential susceptibility to “crazing”, a phenomenon observed either in the form of whitening or cracking.
- opal
- cracking
- water
- TGA
- drying
- Gems
1. Introduction
Opals are well-known gems offering a wide range of aspects and optical phenomena, particularly light diffraction in “noble” opals. The very best specimens reach very high prices in the market. However, the reputation of gem opals is tarnished by its potential to destabilize (or “craze”) in two different ways: It may lose its transparency (“whitening”) or develop fissures (cracking) either at the surface or in its center (examples of cracked opals are shown in Figure 1) [1]. This, of course, alters considerably the value of gem opals. Both phenomena have already been described, but they are not fully understood. Early studies have shown that “whitening“ is correlated with an increase in porosity and a reorganization of the hydration states without changing the silica framework [2,3]. The first empirical investigations on cracking suggest that the drying of opals is a key factor for understanding cracking [4,5,6]. Nevertheless, there remains a need for an experimental or theoretical framework to recognize ahead of time that opals may “craze”. This paper concentrates on the understanding of crazing due to cracking. In order to address the issue of instability in opals and its detection, the materials and their structure and properties must first be understood. Therefore, we first review current knowledge on opal structure, water content, and stability and then experimentally crack a variety of opals to assess the physical origin of this degradation process.
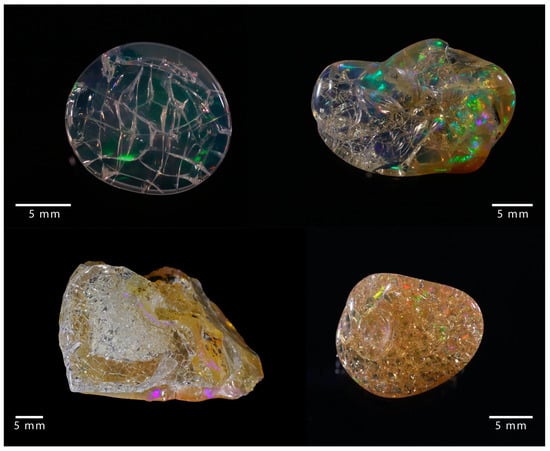
Figure 1. Examples of cracked opal, where a network of fractures developed.
2. Opal
Opal refers to natural hydrated varieties of non- to nanocrystalline silica (SiO2·nH2O) frequently found in a wide range of geological contexts where the aqueous alteration of silicate minerals occurs. A crystallographic classification based on X-ray diffraction (XRD) distinguishes amorphous opal (opal-A) from paracrystalline opal. The latter is further subdivided into opal-CT, where broad cristobalite and tridymite diffraction peaks are observed, and opal-C, where only cristobalite peaks are visible [7]. It has been suggested that opal-CT is a poorly crystallized cristobalite with tridymite stacking [7], but recent structural studies indicated that opal-CT may comprise the nano-domains of diffracting tridymites alternating with non-diffracting silica, with no evidence of cristobalite domains [8,9,10,11]. Opal-A, displaying a broad band typical of amorphous material in XRD, can be subdivided into two sub-types [12]:
The distinction between opal types, even if firstly defined by X-ray diffraction, can also be achieved by Raman spectroscopy [22,23,24], infrared spectroscopy (either in the mid-infrared sensitive to silica framework vibrations [24,25,26] or in the near-infrared sensitive to the hydration state [12,27,28]), nuclear magnetic resonance [29,30], and by observing microstructural features by electron microscopy [9,18,31].
Both opals types are found in various geological contexts. Opal-A is a common phase constituting the silica deposits around hot springs [32,33,34] or during the weathering of rocks [35,36], with a formation temperature ranging from room temperature up to 100 °C and probably more for opal-AN. Similarly, opal-CT can form in a hydrothermal context such as Mexican deposits [37] or by the weathering of volcanic rocks as observed in the Wollo province in Ethiopia [38,39,40] with a formation temperature ranging from ambient up to 160 °C.
Microstructural features observed in opals are all based on nanograins of ca. 25 nm for opal-A [18,41] and of 10 to 50 nm for opal-CT (with an average near 25 nm, including tridymite nano-domains) [18,42,43]. In opal-A, nanograins usually aggregate in spheres ranging from 80 nm to 8 µm in diameter. Opal-CT displays a greater variety of microstructural features where nanograins may accumulate randomly in fibers, platelets, or lepispheres [18,42]. On rare occasions, monodispersed hundred-nanometer-sized silica spheres for opal-A or lepispheres for opal-CT may be arranged into ordered arrays enabling the diffraction of visible light, producing the play-of-colour (POC, patches of moving pure spectral colors) that is highly prized in precious or noble opals [21,44].
3. Water in Opals
In opals, water is present as molecular water (H2O) and chemically bound water in the form of silanol groups (Si-OH) [12,27,45,46,47]. Near-infrared spectroscopy indicates that much of an opal’s hydration consists of molecular water [12,27,48]. Early studies suggested that hydration is concentrated in the interstices between primary spheres [19], but further developments have identified more states in which water is present: interstitial (voids between primary spheres), pore, adsorbed at the surface, and as molecular water trapped in silica cages [12,49,50]. Similarly, silanol groups appear to be located at silica interfaces (such as defects such as broken Si-O-Si bridges in the bulk silica) [12].
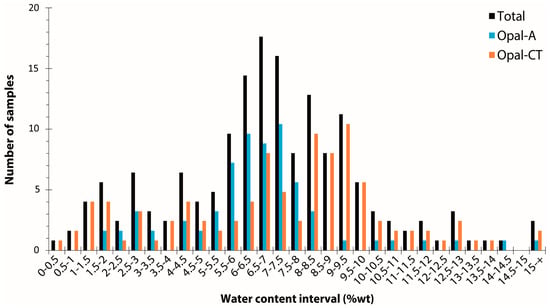
By collating 204 measurements (mostly by thermogravimetric analysis) from the literature [3,6,12,46,51,52,53,54,55,56,57,58,59,60,61], opal water content ranges from 0.5 to 18.1 weight percent, with an average at 6.99 wt% and a standard deviation of 3.05 (Figure 2). A very slight difference between opal-A and opal-CT (6.56 ± 2.41 wt% and 7.32 ± 3.43 wt%, respectively) is shown here between 82 opal-A and 118 opal-CT samples (four opals are not identified). Considering the average and standard deviation, statistically, opal-A and opal-CT are hardly distinguishable based on water content alone. Both opal types have a significant distribution in water content, especially at the lower water content end of the distribution. The opal types do, however, differ in terms of the shape of their distributions: Opal-A shows a distribution centred around 7 wt%, whereas opal-CT has two populations, with one close to opal-A and a second around 9 wt% (Figure 2). In addition, the difference between opals from various origins is statistically indistinguishable: Australia (70 data; 7.31 ± 2.11 wt%), Ethiopia (23 data; 8.43 ± 3.63 wt%), and Mexico (37 data; 8.32 ± 3.54 wt%).
Differential scanning calorimetry (DSC) has demonstrated that between 10 and 33% of the total water present is crystallisable [50,58]. From the melt temperature depression in DSC measurements, it is possible to estimate the pore size in which the crystallisable water is contained. It is estimated to be in the nanometer range (circa 4 to 10 nm in diameter for opal-CT and 6 to 200 (+) nm for opal-A) [50,58]. The crystallisable water contained in opal is present in both isolated and interconnected closed pores or, in some cases, in open pores exposed to the atmosphere, as demonstrated by measurements of the opal’s near-infrared signature at low pressures [62].
4. Instability of Opals by Cracking
In gemmology, instability is defined as an alteration of the aspect hindering the use of the stone for jewellery. Cracking is the development of cracks (fractures) in an initially homogeneous volume, and it obviously affects the integrity of the stone (Figure 3). In an attempt to understand cracking, early studies have attempted to induce cracking empirically, especially by sacrificing stones, although these studies provided no evidence that other samples from the same location would react similarly [4,5,6]. These first investigations revealed that drying rates have a significant impact on the initiation of cracking, suggesting that drying shrinkage in opals could be the main driver for cracking [5,6]. Cracks have been reported to occur primarily in transparent opals, specifically at the surface of the stone [1]. Some attempts have been made to prevent cracking in opals, particularly by applying a specific treatment after mining [63]. Despite a better knowledge of this process, no scientific nor objective criterion has been established at this point to assess or predict the stability of a given sample.

Figure 3. Example of an opal sample collected in 2013 at the mine site (Kok Woha, Wollo Province, Ethiopia, see [39] for details on mines) and the result after 3 years of storage in the atmosphere, where it was allowed to dry.
This entry is adapted from the peer-reviewed paper 10.3390/min13030356
This entry is offline, you can click here to edit this entry!
