
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marcin Dębowski | -- | 2319 | 2024-02-28 10:43:00 | | | |
| 2 | Peter Tang | Meta information modification | 2319 | 2024-02-29 03:43:09 | | |
Video Upload Options
Hydrogen is an environmentally friendly biofuel which, if widely used, could reduce atmospheric carbon dioxide emissions. The main barrier to the widespread use of hydrogen for power generation is the lack of technologically feasible and—more importantly—cost-effective methods of production and storage. Hydrogen has been produced using thermochemical methods (such as gasification, pyrolysis or water electrolysis) and biological methods (most of which involve anaerobic digestion and photofermentation), with conventional fuels, waste or dedicated crop biomass used as a feedstock. Microalgae possess very high photosynthetic efficiency, can rapidly build biomass, and possess other beneficial properties, which is why they are considered to be one of the strongest contenders among biohydrogen production technologies.
1. Introduction
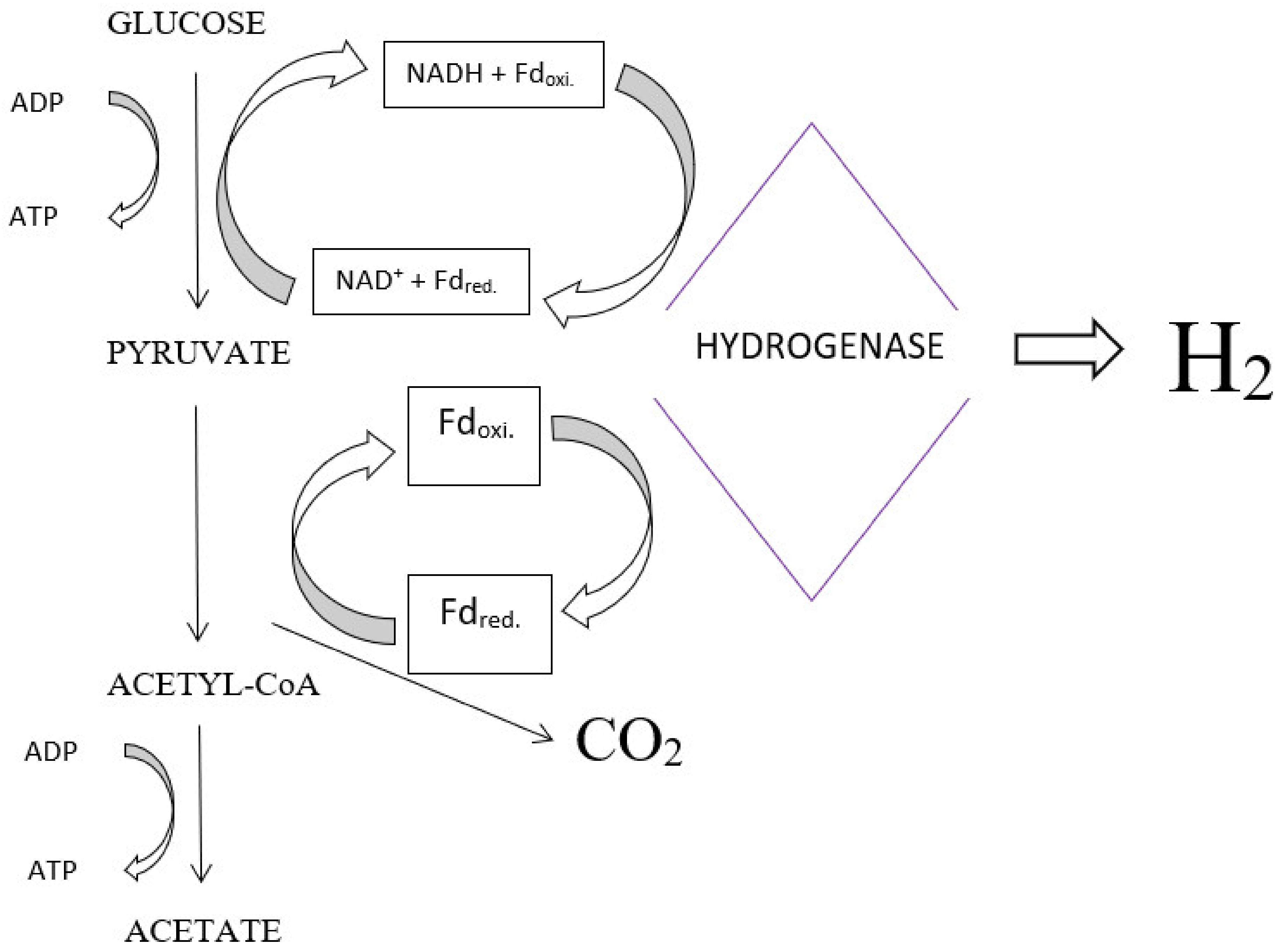
2. Fermentation
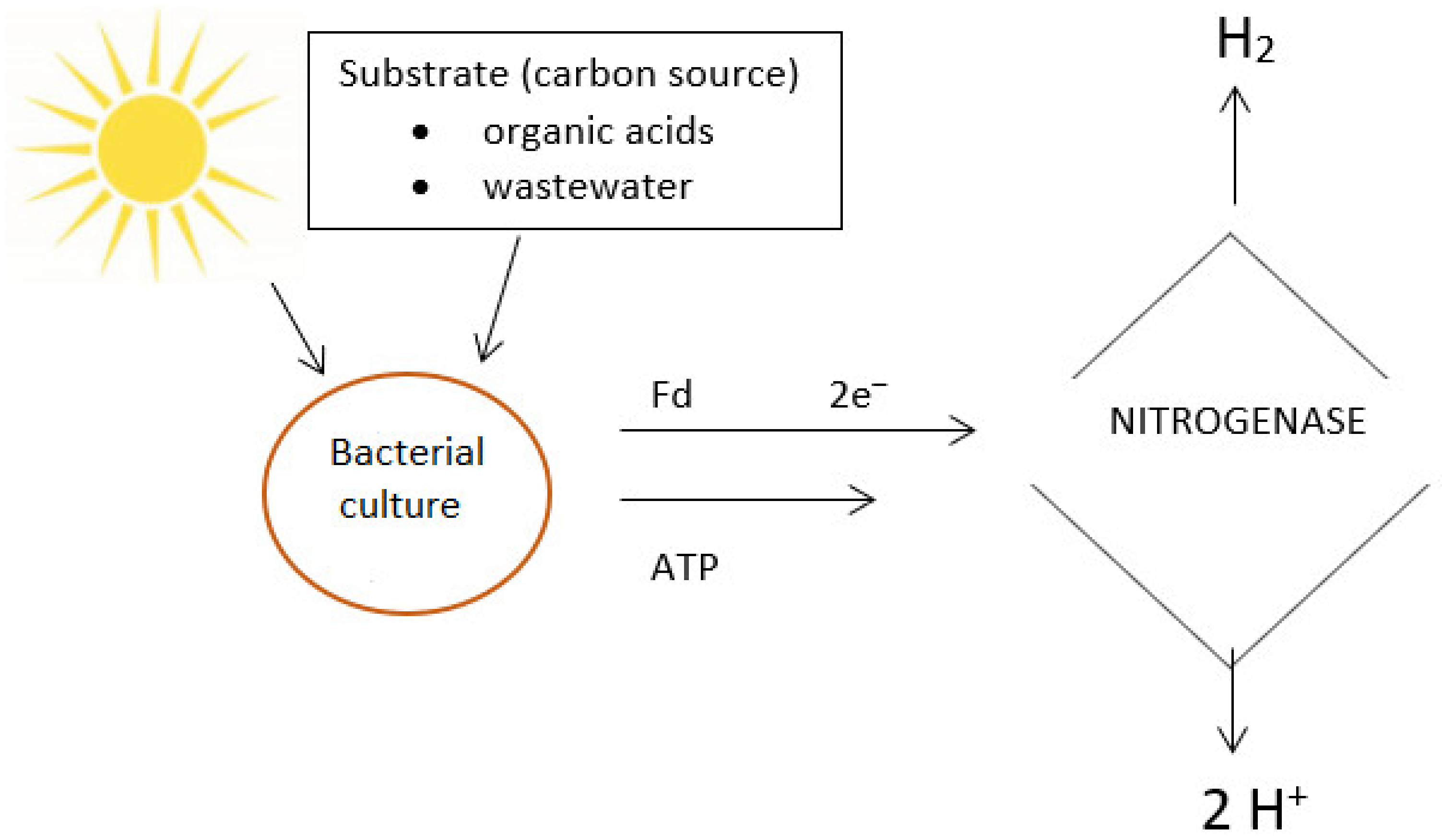
3. Photofermentation
|
Organism |
Substrate |
Light Intensity [W/m2] |
Temp. [°C] |
H2 Production |
Ref. |
|---|---|---|---|---|---|
|
Rhodobacter sphaeroides O.U.001 |
olive mill wastewater |
150 |
30 |
35 dm3 H2/dm3substrate |
[35] |
|
Rhodobacter capsulatus JP91 |
glucose |
175 |
30 |
5.5 mol H2/molglucose |
[47] |
|
Rhodobacter capsulatus |
sucrose (sugar industry molasses) |
200 |
30 |
10.5 mol H2/molsucrose |
[48] |
|
Rhodobacter capsulatus |
sucrose |
200 |
30 |
14 mol H2/molsucrose |
[48] |
|
Rhodobacter capsulatus JY91 |
glucose |
200 |
30 |
3.3 mol H2/molglucose |
[49] |
|
Rhodobacter sphaeroides O.U. 001 |
milk industry wastewater |
116 |
28 |
3.2 dm3 H2/dm3substrate |
[50] |
|
Rhodobium marinum |
soy sauce production wastewater |
240 |
30 |
2.14 molH2/molglucose |
[51] |
|
Rhodobium marinum |
sugar cane bagasse |
240 |
30 |
41 cm3 H2 |
[51] |
|
Rhodobacter capsulatus |
malonate |
200 |
30 |
3.7 mol H2/molsubstrate |
[52] |
|
Rhodobacter capsulatus |
acetate |
200 |
30 |
2.5 mol H2/molsubstrate |
[52] |
References
- Yu, M.; Wang, K.; Vredenburg, V. Insights into low-carbon hydrogen production methods: Green, blue and aqua hydrogen. Int. J. Hydrogen Energy 2021, 46, 21261–21273.
- Meier, K.; Kurtz, C.; Weckerle, C.; Hubner, M.; Bürger, I. Air-conditioning system for vehicles with on-board hydrogen. Appl. Therm. Eng. 2018, 129, 1150–1159.
- Bizon, N.; Raceanu, M.; Koudoumas, E.; Marinoiu, A.; Karapidakis, E.; Carcadea, E. Renewable/Fuel Cell Hybrid Power System Operation Using Two Search Controllers of the Optimal Power Needed on the DC Bus. Energies 2020, 13, 6111.
- Chen, S.; Kumar, A.; Wong, W.C.; Chiu, M.-S.; Wang, X. Hydrogen value chain and fuel cells within hybrid renewable energy systems: Advanced operation and control strategies. Appl. Energy 2019, 233, 321–337.
- Hu, G.; Chen, C.; Lu, H.T.; Wu, Y.; Liu, C.; Tao, L.; Men, Y.; He, G.; Li, K.G. A Review of Technical Advances, Barriers, and Solutions in the Power to Hydrogen (P2H) Roadmap. Engineering 2020, 6, 1364–1380.
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable hydrogen production through biomass gasification: A review and future prospects. Environ. Res. 2020, 186, 109547.
- Chen, J.; Fan, Y.; Jiaqiang, E.; Cao, W.; Zhang, F.; Gong, J.; Liu, G.; Xu, W. Effects analysis on the gasification kinetic characteristics of food waste in supercritical water. Fuel 2019, 241, 94–104.
- Fakeeha, A.; Ibrahim, A.A.; Aljuraywi, H.; Alqahtani, Y.; Alkhodair, A.; Alswaidan, S.; Abasaeed, A.E.; Kasim, S.O.; Mahmud, S.; Al-Fatesh, A.S. Hydrogen production by partial oxidation reforming of methane over Ni catalysts supported on high and low surface area alumina and zirconia. Processes 2020, 8, 499.
- Parkinson, B.; Balcombe, P.; Speirs, J.F.; Hawkes, A.D.; Hellgardt, K. Levelized cost of CO2 mitigation from hydrogen production routes. Energy Environ. Sci. 2019, 12, 19–40.
- Rajabi Hamedani, S.; Villarini, M.; Colantoni, A.; Moretti, M.; Bocci, E. Life Cycle Performance of Hydrogen Production via Agro-Industrial Residue Gasification—A Small Scale Power Plant Study. Energies 2018, 11, 675.
- Shahbaz, M.; Al-Ansari, T.; Aslam, M.; Khan, Z.; Inayat, A.; Athar, M.; Naqvi, S.R.; Ahmed, M.A.; McKay, G. A state of the art review on biomass processing and conversion technologies to produce hydrogen and its recovery via membrane separation. Int. J. Hydrogen Energy 2020, 45, 15166–15195.
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Effect of thermal pretreated organic wastes on the dark fermentative hydrogen production using mixed microbial consortia. Fuel 2021, 284, 119062.
- Tsegaye, B.; Jaiswal, S.; Jaiswal, A.K. Food Waste Biorefinery: Pathway towards Circular Bioeconomy. Foods 2021, 10, 1174.
- Nagarajan, D.; Chang, J.-S.; Lee, D.-J. Pretreatment of microalgal biomass for efficient biohydrogen production—Recent insights and future perspectives. Bioresour. Technol. 2020, 302, 122871–122885.
- Shobana, S.; Kumar, G.; Bakonyi, P.; Saratale, G.D.; Al-Muhtaseb, A.a.H.; Nemestóthy, N.; Bélafi-Bakó, K.; Xia, A.; Chang, J.-S. A review on the biomass pretreatment and inhibitor removal methods as key-steps towards efficient macroalgae-based biohydrogen production. Bioresour. Technol. 2017, 244, 1341–1348.
- Giang, T.T.; Lunprom, S.; Liao, Q.; Reungsang, A.; Salakkam, A. Enhancing Hydrogen Production from Chlorella sp. Biomass by Pre-Hydrolysis with Simultaneous Saccharification and Fermentation (PSSF). Energies 2019, 12, 908.
- Zieliński, M.; Korzeniewska, E.; Filipkowska, Z.; Dębowski, M.; Harnisz, M.; Kwiatkowski, R. Biohydrogen production at low load of organic matter by psychrophilic bacteria. Energy 2017, 134, 1132–1139.
- Dębowski, M.; Korzeniewska, E.; Filipkowska, Z.; Zieliński, M.; Kwiatkowski, R. Possibility of hydrogen production during cheese whey fermentation process by different strains of psychrophilic bacteria. Int. J. Hydrogen Energy 2014, 39, 1972–1978.
- Zhang, H.; Li, J.; Zhang, Q.; Zhu, S.; Yang, S.; Zhang, Z. Effect of Substrate Concentration on Photo-Fermentation Bio-Hydrogen Production Process from Starch-Rich Agricultural Leftovers under Oscillation. Sustainability 2020, 12, 2700.
- Kapdan, I.K.; Kargi, F. Bio-hydrogen production from waste materials. Enzym. Microb. Technol. 2006, 38, 569–582.
- Kumar, N.; Das, D. Enhancement of hydrogen production by Enterobacter cloacae IIT-BT 08. Process. Biochem. 2000, 35, 589–593.
- Kumar, N.; Das, D. Continuous hydrogen production by immobilized Enterobacter cloacae IIT-BT 08 using lignocellulosic ma-terials as solid matrices. Enzym. Microb. Technol. 2001, 29, 280–287.
- Wu, X.; Yao, W.; Zhu, J. Effect of pH on continuous biohydrogen production from liquid swine manure with glucose supple-ment using an anaerobic sequencing batch reactor. Int. J. Hydrogen Energy 2010, 35, 6592–6599.
- Fang, H.H.P.; Liu, H. Effect of pH on hydrogen production from glucose by a mixed culture. Bioresour. Technol. 2002, 82, 87–93.
- Kim, J.K.; Nhat, L.; Chun, Y.N.; Kim, S.W. Hydrogen production conditions from food waste by dark fermentation with Clostridium beijerinckii KCTC 1785. Biotechnol. Bioprocess Eng. 2008, 13, 499–504.
- Song, Z.X.; Dai, Y.; Fan, Q.L.; Li, X.H.; Fan, Y.T.; Hou, H.W. Effects of pretreatment method of natural bacteria source on microbial community and bio-hydrogen production by dark fermentation. Int. J. Hydrogen Energy 2012, 37, 5631–5636.
- Lanzilli, M.; Esercizio, N.; Vastano, M.; Xu, Z.; Nuzzo, G.; Gallo, C.; Manzo, E.; Fontana, A.; D’Ippolito, G. Effect of Cultivation Parameters on Fermentation and Hydrogen Production in the Phylum Thermotogae. Int. J. Mol. Sci. 2021, 22, 341.
- Fabiano, B.; Perego, P. Thermodynamic study and optimization of hydrogen production by Enterobacter aerogenes. Int. J. Hydrogen Energy 2002, 27, 149–156.
- Tenca, A.; Schievano, A.; Perazzolo, F.; Adani, F.; Oberti, R. Biohydrogen from thermophilic co-fermentation of swine manure with fruit and vegetable waste: Maximizing stable production without pH control. Bioresour. Technol. 2011, 102, 8582–8588.
- Xiao, B.; Liu, J. Biological hydrogen production from sterilized sewage sludge by anaerobic self-fermentation. J. Hazard. Mater. 2009, 168, 163–167.
- Chen, W.H.; Chen, S.Y.; Khanal, S.K.; Sung, S. Kinetic study of biological hydrogen production by anaerobic fermentation. Int. J. Hydrogen Energy 2006, 31, 2170–2178.
- Shin, H.S.; Youn, J.H.; Kim, S.H. Hydrogen production from food waste in anaerobic mesophilic and thermophilic acidogenesis. Int. J. Hydrogen Energy 2004, 29, 1355–1363.
- Kisielewska, M.; Dębowski, M.; Zieliński, M. Improvement of biohydrogen production using a reduced pressure fermentation. Bioprocess Biosyst. Eng. 2015, 38, 1925–1933.
- Saye, L.M.G.; Navaratna, T.A.; Chong, J.P.J.; O’Malley, M.K.; Theodorou, M.; Reilly, M. The Anaerobic Fungi: Challenges and Opportunities for Industrial Lignocellulosic Biofuel Production. Microorganisms 2021, 9, 694.
- Eroglu, E.; Gündüz, U.; Yücel, M.; Türker, L.; Eroglu, I. Photobiological hydrogen production from olive mill wastewater as sole substrate sources. Int. J. Hydrogen Energy 2004, 29, 163–171.
- Fan, Q.; Neubauer, P.; Lenz, O.; Gimpel, M. Heterologous Hydrogenase Overproduction Systems for Biotechnology—An Overview. Int. J. Mol. Sci. 2020, 21, 5890.
- Contreras-Angulo, J.R.; Mata, T.M.; Cuellar-Bermudez, S.P.; Caetano, N.S.; Chandra, R.; Garcia-Perez, J.S.; Muylaert, K.; Parra-Saldivar, R. Symbiotic Co-Culture of Scenedesmus sp. and Azospirillum brasilense on N-Deficient Media with Biomass Production for Biofuels. Sustainability 2019, 11, 707.
- Kim, M.S.; Baek, J.S.; Lee, J.K. Comparison of H2 accumulation by Rhodobacter sphaeroides KD131 and its uptake hydrogenase and PHB synthase deficient mutant. Int. J. Hydrogen Energy 2006, 31, 121–127.
- Koku, H.; Eroglu, I.; Gündüz, U.; Yücel, M.; Türker, L. Kinetics of biohydrogen production by the photosynthetic bacterium Rhodobacter spheroids O.U. 001. Int. J. Hydrogen Energy 2003, 28, 381–388.
- Skjånes, K.; Andersen, U.; Heidorn, T.; Borgvang, S.A. Design and construction of a photobioreactor for hydrogen production, including status in the field. Environ. Boil. Fishes 2016, 28, 2205–2223.
- Limongi, A.R.; Viviano, E.; De Luca, M.; Radice, R.P.; Bianco, G.; Martelli, G. Biohydrogen from Microalgae: Production and Applications. Appl. Sci. 2021, 11, 1616.
- Khetkorn, W.; Rastogi, R.P.; Incharoensakdi, A.; Lindblad, P.; Madamwar, D.; Pandey, A.; Larroche, C. Microalgal hydrogen production—A review. Bioresour. Technol. 2017, 243, 1194–1206.
- Yetis, M.; Gündüz, U.; Eroglu, I.; Yücel, M.; Türker, L. Photoproduction of hydrogen from sugar refinery wastewater by Rhodobacter sphaeroides O.U.001. Int. J. Hydrogen Energy 2000, 25, 1035–1041.
- Oh, Y.K.; Scol, E.H.; Kim, M.S.; Park, S. Photoproduction of hydrogen from acetate by a chemoheterotrophic bacterium Rho-dopseudomonas palustris P4. Int. J. Hydrogen Energy 2004, 29, 1115–1121.
- Argun, H.; Kargi, F. Photo-fermentative hydrogen gas production from dark fermentation effluent of ground wheat solution: Effects of light source and light intensity. Int. J. Hydrogen Energy 2010, 35, 1595–1603.
- Laocharoen, S.; Reungsang, A. Isolation, characterization and optimization of photo-hydrogen production conditions by newly isolated Rhodobacter sphaeroides KKU-PS5. Int. J. Hydrogen Energy 2014, 39, 10870–10882.
- Ghosh, D.; Sobro, I.F.; Hallenbeck, P.C. Optimization of the hydrogen yield from single-stage photofermentation of glucose by Rhodobacter capsulatus JP91 using response surface methodology. Bioresour. Technol. 2012, 123, 199–206.
- Keskin, T.; Hallenbeck, P.C. Hydrogen production from sugar industry wastes using single-stage photofermentation. Bioresour. Technol. 2012, 112, 131–136.
- Abo-Hashesh, M.; Ghosh, D.; Tourigny, A.; Taous, A.; Hallenbeck, P.C. Single stage photofermentative hydrogen production from glucose: An attractive alternative to two stage photofermentation or co-culture approaches. Int. J. Hydrogen Energy 2011, 36, 13889–13895.
- Seifert, K.; Waligorska, M.; Laniecki, M. Hydrogen generation in photobiological process from dairy wastewater. Int. J. Hydrogen Energy 2010, 35, 9624–9629.
- Anam, K.; Habibi, M.S.; Harwati, T.U.; Susilaningsih, D. Photofermentative hydrogen production using Rhodobium marinum from bagasse and soy sauce wastewater. Int. J. Hydrogen Energy 2012, 37, 15436–15442.
- Fang, H.H.P.; Liu, H.; Zhang, T. Phototrophic hydrogen production from acetate and butyrate in wastewater. Int. J. Hydrogen Energy 2005, 30, 785–793.
- Sun, Y.; He, J.; Yang, G.; Sun, G.; Sage, V. A Review of the Enhancement of Bio-Hydrogen Generation by Chemicals Addition. Catalysts 2019, 9, 353.
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.-J.; Lee, K.-S.; Lin, C.-Y.; Chen, C.-Y.; Chang, J.-S. Biomass based hydrogen production by dark fermentation—recent trends and opportunities for greener processes. Curr. Opin. Biotechnol. 2018, 50, 136–145.
- Manish, S.; Banerjee, R. Comparison of biohydrogen production processes. Int. J. Hydrogen Energy 2008, 33, 279–286.
- Nath, K.; Kumar, A.; Das, D. Hydrogen production by Rhodobacter sphaeroides strain O.U.001 using spent media of Enterobacter cloacae strain DM11. Appl. Microbiol. Biotechnol. 2005, 68, 533–541.
- Yokoi, H.; Mori, S.; Hirose, J.; Hayashi, S.; Takasaki, Y. H2 production from starch by mixed culture of Clostridium butyricum and Rhodobacter sp. M-19. Biotechnol. Lett. 1998, 20, 895–899.
- Khanal, S.K.; Chen, W.H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131.
- Yokoi, H.; Saitsu, A.S.; Uchida, H.; Hirose, J.; Hayashi, S.; Takasaki, Y. Microbial hydrogen production from sweet potato starch residue. J. Biosci. Bioeng. 2001, 91, 58–63.
- Cheng, J.; Xia, A.; Liu, Y.; Lin, R.; Zhou, J.; Cen, K. Combination of dark- and photo-fermentation to improve hydrogen production from Arthrospira platensis wet biomass with ammonium removal by zeolite. Int. J. Hydrogen Energy 2012, 37, 13330–13337.
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Combination of dark- and photo-fermentation to enhance hydrogen production and energy conversion efficiency. Int. J. Hydrogen Energy 2009, 34, 8846–8853.
- Su, H.; Cheng, J.; Zhou, J.; Song, W.; Cen, K. Improving hydrogen production from cassava starch by combination of dark and photo fermentation. Int. J. Hydrogen Energy 2009, 34, 1780–1786.
- Levin, D.B.; Pitt, L.; Love, M. Biohydrogen production: Prospects and limitations to practical application. Int. J. Hydrogen Energy 2004, 29, 173–185.





