Biomass-based technologies, as well as methods that harness biological processes of microorganisms, are becoming more and more viable as means of hydrogen production
[10][11][10,11]. They consist mostly of organic-feedstock fermentation (carried out by specialised groups of bacteria) and intracellular biochemical processes conducted by certain microalgae species
[12][13][12,13]. Microalgae possess very high photosynthetic efficiency, can rapidly build biomass, are resistant to various contaminants, are amenable to genetic manipulation and can be sited on land that is unsuitable for other purposes. Given these considerations, microalgae seem to represent the most promising route of biohydrogen production
[14][15][14,15].
2. Fermentation
Many authors contend that bacterial fermentation is the most efficient method of converting biomass to hydrogen
[16][17][122,123]. The literature includes accounts of hydrogen-production processes that utilize organic feedstock with various parameters, including organic waste from agricultural, food, meat, and paper industries, as well as livestock manure, slurry, and effluent
[18][19][124,125]. The types of fermentation most crucial to hydrogen production are butyrate/butanol fermentation, typical of the genus
Clostridium sp., and mixed-acid fermentation, mostly used by the family
Enterobacteriaceae (
Escherichia coli,
Enterobacter aerogenes,
Klebsiella pneumoniae,
Vibrio cholerae,
Shigella dysenteriae) and
Bacillus sp.
[20][126].
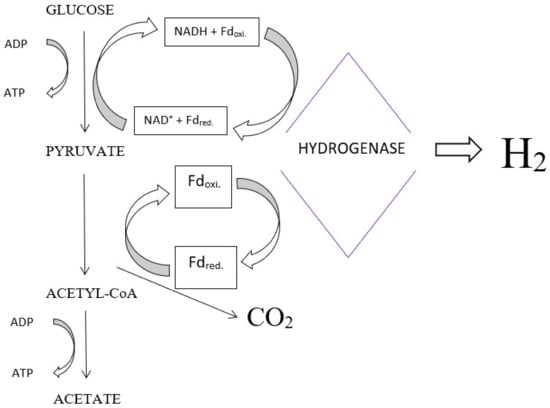
The mechanism of biohydrogen formation in anaerobic processes involves the reduction of protons by hydrogenase, using electrons donated by ferredoxin. The electrons are released by the degradation of glucose to pyruvate, which is then oxidised to acetyl-CoA and CO
2. A diagram of an example biohydrogen production by
Clostridium sp. bacteria is presented in
Figure 1.
Figure 1.
Diagram of hydrogen production by
Clostridium
sp.
Kumar and Das
[21][22][127,128] provide a detailed outline of hydrogen production mechanisms via fermentation by
Enterobacter cloacae IIT—BT 08, using different sources of organic matter. Using sucrose and cellobiose as feedstock led to the highest hydrogen yields at 6.0 H
2 mol/mol substrate and 5.4 H
2 mol/mol substrate, respectively, with a production rate of 35.6 mmol H
2/dm
3·h. The authors surpassed this production rate in a different study, reaching 75.6 mmol H
2/dm
3·h. In this case, the reactor used to grow anaerobic bacteria was packed with lignocellulosic materials, including rice straw, bagasse, and coconut coir. The reactor packed with coir performed the best. The authors attribute this finding to the higher cell density in the coir matrix, perhaps due to the largest active surface area available to cells. A study by Wu et al. investigated fermentation of swine manure supplemented with glucose. Hydrogen production was 2.25 dm
3/dm
3·d, whereas the hydrogen content of biogas peaked at 36.9%. The authors also looked at optimum pH for fermentation and noted the best performance at pH 5.0, which ensured stable hydrogen production and concentration throughout the experiment (22 d). The experiment used an ASBR system, with a glucose degradation efficiency ranging from 98.5 to 99.6%
[23][129]. Fang and Liu tested a dark hydrogen fermentation process in a 3 L reactor at a pH range of 4.0–7.0. A synthetic medium with 7.0 g/dm
3 glucose was fed into a digester. The process turned stable after 14 days and degraded 90% of the glucose. The hydrogen yields at optimal pH (5.5) reached 2.1 mol H
2/mol glucose with 64% hydrogen content in the biogas
[24][130]. Kim et al. achieved a hydrogen production of 128 cm
3/g COD
removed. The hydrogen yield was close to 110 cm
3/dm
3·h. Food waste was fermented using
Clostridium beijerinckii KCTC 1785. The process was run at pH 5.5 and 40 °C
[25][131]. Song et al. used cow dung compost for dark fermentation and obtained a hydrogen yield of 290.8 cm
3/dm
3 culture. The feedstock input into the system had a concentration of 10 g/dm
3. Initial pH was around 7.0. The dominant hydrogen producers were
Clostridium sp. and
Enterobacter sp.
[26][132].
Fermentative hydrogen production is influenced by many factors and system parameters, including substrate type, substrate concentration, hydraulic retention time (HRT), type of digester, pH, temperature and microbial strain
[27][133]. Even trace amounts of oxygen in the system inhibit hydrogenase activity in obligate anaerobes, which is why it is usually the safer choice to use facultative anaerobes, with
Clostridium sp. and
Enterobacter sp. being the most common. This is due to the fact that these bacteria better tolerate oxygen in bioreactors
[28][134]. Optimal pH for efficient hydrogen production ranges from 5.0 to 6.0. Lower values have a direct effect in switching microbial metabolism towards biochemical processes that lead to different make-up of the resultant gas and decreased hydrogen production. Furthermore, pH under 4.0 can inhibit microbial growth
[23][129]. Conversely, increased pH induces methanogenic bacteria to grow, consuming hydrogen to produce methane
[29][135]. One simple technological procedure, commonly used to eliminate methanogenic bacteria from the communities in anaerobic sludge, is to perform heat treatment at 80–104 °C
[30][136]. Heat conditioning of anaerobic microflora ensures the survival of hydrogenous spore-forming microbes, including
Clostridium sp. and
Bacillus sp.
[31][137]. Short hydraulic retention times (12 h or less) can also be used to limit the growth of bacteria that compete with hydrogen-producing microbes
[32][138]. The efficiency of hydrogen fermentation can be hampered by undissociated volatile fatty acids generated in the reactor
[29][135]. Partial pressure of gaseous hydrogen has also been identified as an important factor. Excessive hydrogen levels in the system lead to accumulation of propionic acid and butyric acid, reducing hydrogen production. Reducing the pressure—and thus, the hydrogen concentration—can significantly improve performance
[33][139]. Other parameters are also important, including substrate profile, nitrogen levels (used as a nutrient by microorganisms) or iron levels (involved in hydrogenase activity). According to literature data, iron levels should be kept between 10 and 100 mg/dm
3 [20][126].
3. Photofermentation
Photofermentation is done by anaerobic bacteria capable of converting organic acids to hydrogen and CO
2 [34][140]. Most common photosynthesizers include green/purple sulphur and non-sulphur bacteria. The species most widely covered in the literature are:
Rhodobacter spheroides,
Rhodobacter capsulatus,
Rhodobacter sulidophilu,
Rhodopseudomonas palustris,
Rhodopseudomonos sphaeroides, and
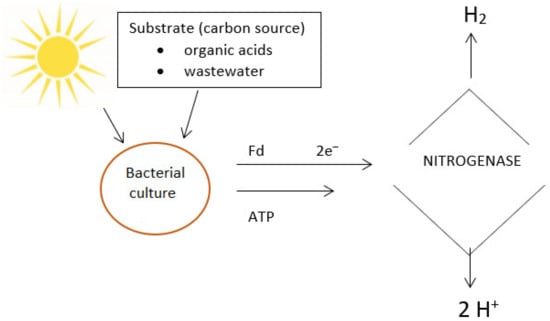
Halobacterium haobium [35][141]. Nitrogenase is the primary photofermentative enzyme, capable of catalyzing its reaction in either direction
[36][142]. In the presence of inhibitory nitrogen, electrons are transferred by ferredoxin and used by microorganisms to reduce molecular nitrogen into ammonia. In a nitrogen-deprived environment, electrons carried by nitrogenase reduce protons into molecular hydrogen. Nitrogenase activity can be inhibited by oxygen, ammonia or excessive C/N
[37][143]. The biochemistry of photofermentative hydrogen production involves the transfer of electrons released during the decomposition of organic substrate by ferredoxin, which are taken up by nitrogenase and used to reduce protons to molecular hydrogen. The energy necessary for protein-mediated electron transfer is derived from the light source. A diagram of the photofermentation process is presented in
Figure 2.
Figure 2.
Diagram of photofermentative hydrogen production by microbes.
Achieving suitable conditions for photofermentation requires a source of bright 400–1000 nm light (6–10 klux), a temperature between 30 °C and 36 °C, and near-neutral pH (6.8–7.5)
[38][144]. Optimal illumination intensity boosts hydrogen production rates and yields. However, due to the high running costs of such a solution, alternating light-dark cycles are usually used instead. The cycles tend to be equal in length, 12/12 h being the most common regime
[39][145]. Hydrogen yields produced through photofermentation are also largely and directly determined by the type and design of the bioreactor
[40][146]. Tubular, column and flat-plate reactors are the most common
[41][147]. These units are typically closed and hermetically sealed, preventing contamination, oxygen penetration and growth of competing microbial species. Bioreactors currently used for photofermentation are similar in design to those used for cultivating and growing microalgae
[42][148].
Yetis et al. studied photofermentative hydrogen production by
Rhodobacter spheroides O.U.001 using sugar refinery wastewater as feedstock. The hydrogen yield was 3.8 cm
3/dm
3·h. When malic acid was added to the feedstock, production rate increased to 5.0 cm
3/dm
3·h
[43][149]. Eroglu et al. demonstrated that colored and organic-rich wastewater needs to be diluted. Dilution of olive mill wastewater led to hydrogen production of 13.9 dm
3 H
2/dm
3 by
Rhodobacter sphaeroides O.U.001
[35][141]. Oh et al. used
Rhodopseudomonas palustris P4 for photofermentation, producing 2.4–2.8 mol H
2/mol acetic acid
[44][150]. Argun and Kargi investigated the effect of different light sources and intensities on photofermentative hydrogen production by
Rhodobacter sphaeroides–RV. Fatty acids derived from ground wheat starch served as the feedstock. Tests with halogen lamps led to the highest hydrogen production of 252 cm
3 and production efficiency of 781 cm
3 H
2/g fatty acids. A light intensity of 5 klux provided the best performance in terms of hydrogen production at 1037 cm
3/g fatty acids
[45][151]. Laocharoen and Reungsang ran a photofermentative process using
Rhodobacter sphaeroides KKU– PS5. The operational parameters of the fermentation were as follows: temperature—30 °C; light intensity—6 klux; initial pH—7.0. Malic acid at a concentration of 30 mmol/dm
3 served as the primary feedstock for the microorganisms. Hydrogen production yields and rates were 1330 cm
3 H
2/dm
3, 3.80 mol/mol malate, and 11.08 cm
3 H
2/dm
3·h respectively
[46][152]. The efficiencies of photofermentative biohydrogen production, according to literature data, are presented in
Table 1.
Table 1.
Comparison of literature data on photofermentative hydrogen productivity.
Literature data indicate that a combination of dark and photofermentation is a valid method of enhancing hydrogen production
[53][153]. Dark fermentation produces organic acids and alcohols as by-products, which serve as a carbon source for the bacteria involved in photofermentative hydrogen production
[54][154]. Integrated biological processes significantly improve the ratio of energy stored in the hydrogen to the energy needed to maintain the culture. This ratio was 3.0 in studies by Manish and Banerjee
[55][155].
Nath et al. applied glucose for hydrogen production by
Enterobacter cloacae strain DM11, with dark fermentation as the first stage of the process. The gas yield amounted to 1.86 mol H
2/mol glucose. The spent medium from the fermentation, which mainly contained acetic acid, was then used as a source of carbon for photofermentation by
Rhodobacter sphaeroides O.U.001, which resulted in an additional 1.5 to 1.72 mol H
2/mol of acetic acid
[56][156]. Yokoi et al. increased the gas production twofold by using
Clostridium butyricum and
Rhodobacter sp. M-19, with a total yield of 6.6 mol H
2/mol glucose
[57][157].
Khanal et al. produced 7.2 mol H
2/mol glucose by using starch production waste as the basic feedstock for fermentation, and photofermented the residue (which contained lactic, butyric and acetic acids)
[58][164]. Yokoi et al. have produced similar results using
Clostridium butricum, Enterobacter aerogenes, and
Rhodobacter sp. M-19 grown on potato-processing waste
[59][165]. Cheng et al. conducted a two-stage hydrogen production process using
Arthrospira platensis pre-treated with microwaves and H
2SO
4. Dark fermentation of the feedstock (containing 10 g/dm
3 glucose) resulted in a hydrogen yield of 96.6 cm
3/g dry matter. The spent solution had a high NH
4+ content of 31.6–96.6 mM. This residue was then treated by ion exchange to prevent interference with subsequent biochemical processes, removing 91.8–95.8% ammonium ions. The treated residue could then be further photofermented, resulting in hydrogen production of 240.4 cm
3/g dry matter. The entire system produced a total of 337 cm
3 H
2 per gram of dry matter feedstock
[60][166].
The performance of hybrid systems was also examined by Su et al., who set up an integrated dark and photo fermentation process with cassava starch as the substrate (10–25 g/dm
3 concentration). The dark fermentation stage produced 240.0 cm
3 hydrogen per gram of starch at a concentration of 10 g/dm
3, with the maximum production rate being 84.4 cm
3 H
2/dm
3·h at a concentration of 25 g/dm
3. The by-products of this stage—acetate and butyrate—were then used as substrates for fermentation in the presence of
Rhodopseudomonas palustris. The yield at this stage amounted to 131.9 cm
3 H
2/g starch at a rate of 16.4 cm
3 H
2/dm
3·h. Acetate and butyrate conversion capacities were respectively 89.3% and 98.5%. The total hydrogen yield of the two-stage process was 402.3 cm
3/g starch
[61][167]. In another study, Su et al. presented a method for improving hydrogen yields in a two-stage process. Hydrogen production during the dark fermentation stage reached 1.72 mol/mol glucose at a rate of 100 cm
3 H
2/dm
3·h. In the photofermentation stage, the yield was 4.16 mol/mol glucose. Metabolites from the dark cycle were converted in this stage with an efficiency of 92.3% (acetate) and 99.8% (butyrate). The two-step process yielded 5.48 mol H
2/mol glucose
[62][168].
There has been an increasingly prevalent opinion that hybrid systems are capable of producing 12 mol H
2/mol glucose at their peak, which had once been considered a purely theoretical threshold
[63][169].