Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Soumitra Das | -- | 1895 | 2024-02-28 01:36:41 | | | |
| 2 | Lindsay Dong | -22 word(s) | 1873 | 2024-02-28 06:42:13 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhao, W.; Kodancha, P.; Das, S. Gut Microbiome Changes in Anorexia Nervosa. Encyclopedia. Available online: https://encyclopedia.pub/entry/55583 (accessed on 07 February 2026).
Zhao W, Kodancha P, Das S. Gut Microbiome Changes in Anorexia Nervosa. Encyclopedia. Available at: https://encyclopedia.pub/entry/55583. Accessed February 07, 2026.
Zhao, Wendi, Prabhath Kodancha, Soumitra Das. "Gut Microbiome Changes in Anorexia Nervosa" Encyclopedia, https://encyclopedia.pub/entry/55583 (accessed February 07, 2026).
Zhao, W., Kodancha, P., & Das, S. (2024, February 28). Gut Microbiome Changes in Anorexia Nervosa. In Encyclopedia. https://encyclopedia.pub/entry/55583
Zhao, Wendi, et al. "Gut Microbiome Changes in Anorexia Nervosa." Encyclopedia. Web. 28 February, 2024.
Copy Citation
Anorexia nervosa (AN) remains a challenging condition in psychiatric management and its pathogenesis is not yet fully understood. An imbalance in the gut microbiota composition may contribute to its pathophysiology. There is an imbalance in gut microbiota composition leads to reduced short-chain fatty acids, contributing to a proinflammatory state in AN, which is also common in other psychiatric comorbidities. Microbial changes may also contribute to the semistarvation state through endocrine changes and altered energy utilization.
anorexia nervosa
gut microbiome
microbiota
1. Introduction
Anorexia nervosa (AN) remains a challenging condition in psychiatric management, as the mortality rate is the highest among psychiatric disorders and can be relapsing or chronic in its course [1][2]. Less than half of the patients (46%) fully recover from AN, and it is commonly complicated by other psychiatric comorbidities such as affective disorders (1/4 AN patients) and anxiety disorders (1/4 AN patients) [2][3]. The highly comorbid nature of AN makes it difficult to discern if any gut microbiome changes are unique to AN alone or common in other psychiatric conditions.
The pathogenesis is multifactorial and still not fully understood since it manifests in multiple non-CNS organ systems, such as immunological and endocrine dysfunction [4]. AN has a heritability component ranging from 28 to 74% and eight significant loci implicated in AN development have been identified [4][5]. Individual traits such as anxiety, perfectionism, and obsessive-compulsivity are both risk and prognostic factors [6].
AN patients typically have chronic caloric restriction, macronutrient and micronutrient deficiencies, changing food availability, and high fibre intake [7]. Genetics, infection, and inflammation can all directly affect dysbiosis, though profound changes in gut microbiota may be a result of changes in macronutrients [8]. The interlinking of multiple variables that affect dysbiosis highlights a limitation in investigating the role of dysbiosis in AN pathogenesis, whether it contributes to the maintenance or precedes the onset of AN.
AN patients are significantly underweight and require nutritional rehabilitation that comes with the potentially fatal risk of refeeding syndrome [4]. Current identification of high-risk refeeding syndrome patients follows guidelines developed by the National Institute for Health and Clinical Excellence (NICE) [9], and another screening test is the Short Nutritional Assessment Questionnaire [10]. However, these existing strategies to identify at-risk patients remain poorly validated, especially for predicting severe hypophosphatemia, a key characteristic of refeeding syndrome [11].
2. Gut Microbiome Changes in Anorexia Nervosa
2.1. Changes in Gut Microbiome Composition
2.1.1. Alpha Diversity
In microbiology, alpha diversity estimates the diversity within a single community, comprising the number of species present (richness) and the distribution of the number of organisms per species (evenness), i.e., their relative abundance and taxonomic distribution [12]. The diversity of a community is highly related to its environment and decreases in the setting of environmental changes (e.g., from a healthy to a diseased state). For example, alpha diversity is often decreased in irritable bowel syndrome [13]. In addition to observing decreased alpha diversity in AN, two studies observed an increase in alpha diversity in post-weight-restoration patients compared to before intervention [14][15], suggesting that low microbial diversity is implicated in lower BMI and greater starvation severity in AN. However, reduced alpha diversity is not a finding specific to AN, as it is also observed in AN patients with depression or anxiety. Depression is a prominent psychiatric feature secondary to the sequelae of semistarvation in AN [1], so it is possible that reduced alpha diversity may represent a more severe disease pathology [16]. However, there were inconsistencies in the alpha diversity changes in AN patients compared to HCs [17][18][19][20][21], and post- compared to pre-weight-restoration [16]. Differences in the size of patients’ stool samples and sample analysis techniques may explain these discrepancies. Furthermore, the discrepancy in alpha diversity data may be explained by the differences in the measurement indices (Shannon, Chao, Fischer, etc.)—there does not exist an absolute measure of diversity and each method has its own biases and advantages. Hence, the lack of specificity in choosing appropriate methods can lead to the oversimplification of diversity outcomes [22]. For example, the role of alpha diversity in general disease pathogenesis remains inconsistent, as studies in other disease states report an increase in alpha diversity [23][24].
Hence, it may be more useful to postulate the role of gut microbiota changes in AN pathogenesis by scrutinising individual taxa; however, this comes with another set of limitations. There is an inherent limitation in the interpretation of bacterial relative abundance for clinical analysis, as compositional data are not independent of each other and different biological scenarios can yield the same proportions of taxa over changes in time [25]. Reference frames have been identified as a way to alleviate false positives [25]. The reliability of compositional analysis is limited to the resolution of the sequencing method—16S rRNA sequencing or shotgun metagenomics and metagenomics relies on the known genomes of gut microorganisms [26].
2.1.2. Decreased Faecalibacterium prausnitzii
AN microbial communities were consistently depleted in Faecalibacterium [14][16][19][21] compared to HCs, and one study found a negative correlation between eating disorder scores and Faecalibacterium prausnitzii [27]. Faecalibacterium metabolise dietary fibres and other complex carbohydrates to produce short-chain fatty acids (SCFAs), such as butyrate [28]. SCFAs maintain the integrity of the intestinal barrier [29], promote immune cell recruitment to the gut, and increase the production of inflammatory mediators [30]. One type of SCFA is butyrate, and Faecalibacterium prausnitzii is the most common butyrate-producing species in faecal samples (5% abundance in healthy stool) [28]. Butyrate maintains the immunological aspect of gut barrier integrity by regulating Claudin-1 and synaptopodin expression, limiting pro-inflammatory cytokines (IL-6, IL-12), and inhibiting oncogenic pathways [28]. The synthesis of proinflammatory IL-6 and IL-12 is inhibited through the action of Faecalibacterium prausnitzii components inducing the IL-10 production in immune cells [31]. In addition to maintaining healthy intestinal barrier integrity, butyrate produced by Faecalibacterium prausnitzii also plays an important role in restricting the entry and establishment of pathogenic microbes [32]. Butyrate activates PPAR-ɣ signalling, which drives the high-oxygen-consuming metabolism within colonocytes, maintaining a state of epithelial hypoxia [32]. The anaerobic environment in the gut lumen, maintained by butyrate-producing bacteria, prevents colonisation by pathogenic Salmonella and E. coli [33]. Hence, in all these ways butyrate normally modulates the inflammatory responses within the gut. However, where these butyrate-producing species are decreased, authors hypothesize that there exists a pro-inflammatory state in the gut that contributes to AN (Figure 1).
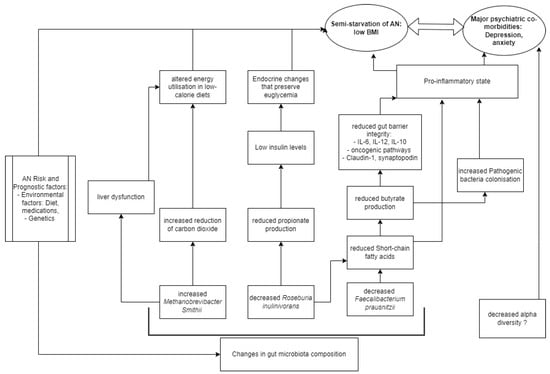
Figure 1. Model of AN pathogenesis related to gut microbiota.
There is a growing body of evidence to show that depression is associated with a chronic, low-grade inflammatory response and activation of cell-mediated immunity [34], similar to the speculation on the proinflammatory state in AN pathogenesis. The disrupted host inflammatory responses may be a result of butyrate or other SCFAs, as there is evidence to support that several SCFA-producing species (Bifidobacterium, Faecalibacterium, Lactobacillus) are reduced in depression and anxiety [34][35]. A negative correlation was observed between Faecalibacterium and the severity of depressive symptoms in a cross-sectional study [34]. Hence, a reduction in Faecalibacterium abundance is a finding non-specific to AN compared to other psychiatric disorders, but its abundance may negatively correlate to severe psychiatric disease [27].
2.1.3. Decreased Roseburia inulinivorans
Roseburia, another butyrate-producing Firmicute of similar significance, was consistently depleted [17][18][27][36], and negatively correlated with Eating Disorder Examination (17th edition) scores [15]. Levels of insulin are known to be reduced in AN patients compared to HCs, a phenomenon that helps preserve euglycemia by reducing cellular glucose uptake and glycogenesis [37], and the possible link to Roseburia species may be in propionate. Propionate, which is produced by Roseburia inulinivorans from fucose [38], has been shown to directly stimulate insulin secretion via protein kinase C and protect beta cells from apoptotic stimuli in the long term [39]. Decreased propionate levels in AN were observed in two studies [17][40], suggesting that the effective impacts of altered gut bacteria are endocrinological as well as neuro-inflammatory and immunological in contributing to the semistarvation state of AN (Figure 1).
2.1.4. Increased Methanobrevibacter smithii
Another consistent alteration in AN microbiome composition is the enrichment of Methanobrevibacter smithii [17][18][41][42], which is already well-documented as representing an adaptive response to prolonged caloric restriction [43]. Methanobrevibacter smithii uses hydrogen to reduce carbon dioxide to methane, allowing for optimal nutrient transformation in very low-calorie diets [41]. However, higher levels of the archaea have also been found in obesity, constipation, and irritable bowel syndrome [44], as well as non-alcoholic fatty liver disease and cirrhosis [17]. Borgo et al. also confirmed previously described increases in liver enzymes (ALT and AST) compared to HCs [17]. Hence, it is possible that Methanobrevibacter smithii contributes to altered metabolism, through a disruption of liver function commonly seen in AN patients (Figure 1). The contribution of Methanobrevibacter smithii to the semistarvation of AN is supported by Million et al., who observed a negative correlation between BMI and Methanobrevibacter smithii [42].
2.1.5. Comparison to Other Existing Pathophysiological Models
Gabriel et al. observed a chronic AN cohort (average illness duration 5.5 years) with a non-inflammatory cytokine profile; similarly, Nisson et al. studied a chronic AN cohort (average illness duration 10.8 years) with non-high levels of IL-6 in AN patients compared to HCs [45][46]. A meta-analysis of shorter-AN-duration cohorts showed elevated cytokine levels compared to HCs for IL-1beta, TNF-alpha, and IL-6; it appears that immune status varies according to AN disease duration [47][48]. This raises the limitation that most of the studies did not specify the time from the onset of disease, which means that the proinflammatory model in AN (Figure 1) is limited to the assumption of acute disease. In a review of genetic risk factors for eating disorders, Himmerich et al. highlighted an additional genetic role of SCFAs, as butyrate is an HDAC inhibitor with potential effects on gene expression in human cells [49].
Another hypothesis suggests that the energy needs of the gut microbiome may regulate the aberrant eating behaviour of individuals with AN; bacteria may produce modules that regulate the production of neurohormones involved in mood and eating behaviour or act directly as neurohormone-like molecules [50]. This describes a different link between SCFAs and AN, whereby SCFAs can directly act on enteroendocrine cells of the intestinal epithelium and activate the release of hormones contributing to satiation such as peptide tyrosine tyrosine or glucagon-like peptide 1 [50]. Researchers are increasingly focusing on the Enterobacteriaceae ClpB protein (caseinolytic peptidase B protein homologue) that can mimic the alpha-melanocyte-stimulating hormone involved in appetite control [50][51]. Three studies have found an increased prevalence of Clp-B-producing bacteria in AN patients [17][36][42].
2.2. Refeeding Syndrome
There have been numerous systematic reviews in recent years surrounding refeeding syndrome management in AN. Faecalibacterium, Roseburia and other butyrate-producing Firmicutes ferment dietary fibre and are consistently depleted in AN patients relative to healthy controls. Studies observed gut microbial composition post-weight-restoration, of which two are known to provide high-calorie diets (high in protein, fat, and predominantly carbohydrate) resulting in an increase in alpha diversity compared to before refeeding [14][15]. In contrast, only one study utilised a high-fibre, high-fat, and high-energy diet that resulted in decreased Bacteroidetes and increased Firmicutes compared to before refeeding [18]. The increase in Firmicutes is unlikely to be due to the high fibre consumption as total SCFA concentrations do not differ after refeeding; rather, it is likely attributable to the high fat or carbohydrate intake [52].
3. Conclusions
There is consistent evidence to support changes in gut microbiome composition in AN patients compared to health-weight controls, yet it remains unclear whether these changes contribute to the maintenance or precede the onset of AN. No results were obtained for refeeding syndrome. Microbial community changes in AN manifest in reduced anti-inflammatory effects at the level of the intestinal barrier, implicating a pro-inflammatory state in AN. However, this state is non-specific to AN compared to other psychiatric disorders.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing, Inc.: Arlington, VA, USA, 2013.
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality Rates in Patients With Anorexia Nervosa and Other Eating Disorders: A Meta-analysis of 36 Studies. Arch. Gen. Psychiatry 2011, 68, 724–731.
- Hans-Christoph Steinhausen, M.D. The Outcome of Anorexia Nervosa in the 20th Century. Am. J. Psychiatry 2002, 159, 1284–1293.
- Zipfel, S.; Giel, K.E.; Bulik, C.M.; Hay, P.; Schmidt, U. Anorexia nervosa: Aetiology, assessment, and treatment. Lancet Psychiatry 2015, 2, 1099–1111.
- Watson, H.J.; Yilmaz, Z.; Thornton, L.M.; Hübel, C.; Coleman, J.R.I.; Gaspar, H.A.; Bryois, J.; Hinney, A.; Leppä, V.M.; Mattheisen, M.; et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat. Genet. 2019, 51, 1207–1214.
- Barakat, S.; McLean, S.A.; Bryant, E.; Le, A.; Marks, P.; Aouad, P.; Barakat, S.; Boakes, R.; Brennan, L.; Bryant, E.; et al. Risk factors for eating disorders: Findings from a rapid review. J. Eat. Disord. 2023, 11, 8.
- Bulik, C.M.; Flatt, R.; Abbaspour, A.; Carroll, I. Reconceptualizing anorexia nervosa. Psychiatry Clin. Neurosci. 2019, 73, 518–525.
- Hrncir, T. Gut Microbiota Dysbiosis: Triggers, Consequences, Diagnostic and Therapeutic Options. Microorganisms 2022, 10, 578.
- NICE. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition; National Institute for Health and Care Excellence (NICE): London, UK, 2017.
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 2005, 24, 75–82.
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195.
- Xia, Y.; Sun, J. Alpha Diversity. In Bioinformatic and Statistical Analysis of Microbiome Data: From Raw Sequences to Advanced Modeling with QIIME 2 and R; Xia, Y., Sun, J., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 289–333.
- Kelly, J.; Kennedy, P.; Cryan, J.; Dinan, T.; Clarke, G.; Hyland, N. Breaking Down the Barriers: The Gut Microbiome, Intestinal Permeability and Stress-related Psychiatric Disorders. Front. Cell. Neurosci. 2015, 9, 392.
- Fouladi, F.; Bulik-Sullivan, E.C.; Glenny, E.M.; Thornton, L.M.; Reed, K.K.; Thomas, S.; Kleiman, S.; Watters, A.; Oakes, J.; Huh, E.-Y.; et al. Reproducible changes in the anorexia nervosa gut microbiota following inpatient therapy remain distinct from non-eating disorder controls. Gut Microbes 2022, 14, 2143217.
- Monteleone, A.M.; Troisi, J.; Fasano, A.; Dalle Grave, R.; Marciello, F.; Serena, G.; Calugi, S.; Scala, G.; Corrivetti, G.; Cascino, G.; et al. Multi-omics data integration in anorexia nervosa patients before and after weight regain: A microbiome-metabolomics investigation. Clin. Nutr. 2021, 40, 1137–1146.
- Kleiman, S.C.; Carroll, I.M.; Tarantino, L.M.; Bulik, C.M. Gut feelings: A role for the intestinal microbiota in anorexia nervosa? Int. J. Eat. Disord. 2015, 48, 449–451.
- Borgo, F.; Riva, A.; Benetti, A.; Casiraghi, M.C.; Bertelli, S.; Garbossa, S.; Anselmetti, S.; Scarone, S.; Pontiroli, A.E.; Morace, G.; et al. Microbiota in anorexia nervosa: The triangle between bacterial species, metabolites and psychological tests. PLoS ONE 2017, 12, e0179739.
- Mack, I.; Cuntz, U.; Gramer, C.; Niedermaier, S.; Pohl, C.; Schwiertz, A.; Zimmermann, K.; Zipfel, S.; Enck, P.; Penders, J. Weight gain in anorexia nervosa does not ameliorate the faecal microbiota, branched chain fatty acid profiles, and gastrointestinal complaints. Sci. Rep. 2016, 6, 26752.
- Prochazkova, P.; Roubalova, R.; Dvorak, J.; Kreisinger, J.; Hill, M.; Tlaskalova-Hogenova, H.; Tomasova, P.; Pelantova, H.; Cermakova, M.; Kuzma, M.; et al. The intestinal microbiota and metabolites in patients with anorexia nervosa. Gut Microbes 2021, 13, 1902771.
- Schulz, N.; Belheouane, M.; Dahmen, B.; Ruan, V.A.; Specht, H.E.; Dempfle, A.; Herpertz-Dahlmann, B.; Baines, J.F.; Seitz, J. Gut microbiota alteration in adolescent anorexia nervosa does not normalize with short-term weight restoration. Int. J. Eat. Disord. 2021, 54, 969–980.
- Yuan, R.; Yang, L.; Yao, G.; Geng, S.; Ge, Q.; Bo, S.; Li, X. Features of gut microbiota in patients with anorexia nervosa. Chin. Med. J. 2022, 135, 1993–2002.
- Shade, A. Diversity is the question, not the answer. ISME J. 2017, 11, 1–6.
- Coretti, L.; Paparo, L.; Riccio, M.P.; Amato, F.; Cuomo, M.; Natale, A.; Borrelli, L.; Corrado, G.; De Caro, C.; Comegna, M.; et al. Gut Microbiota Features in Young Children with Autism Spectrum Disorders. Front. Microbiol. 2018, 9, 3146.
- Yin, J.; Liao, S.X.; He, Y.; Wang, S.; Xia, G.H.; Liu, F.T.; Zhu, J.J.; You, C.; Chen, Q.; Zhou, L.; et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J. Am. Heart Assoc. 2015, 4, e002699.
- Morton, J.T.; Marotz, C.; Washburne, A.; Silverman, J.; Zaramela, L.S.; Edlund, A.; Zengler, K.; Knight, R. Establishing microbial composition measurement standards with reference frames. Nat. Commun. 2019, 10, 2719.
- Allaband, C.; McDonald, D.; Vázquez-Baeza, Y.; Minich, J.J.; Tripathi, A.; Brenner, D.A.; Loomba, R.; Smarr, L.; Sandborn, W.J.; Schnabl, B.; et al. Microbiome 101: Studying, Analyzing, and Interpreting Gut Microbiome Data for Clinicians. Clin. Gastroenterol. Hepatol. 2019, 17, 218–230.
- Fan, Y.; Stoving, R.K.; Berreira Ibraim, S.; Hyotylainen, T.; Thirion, F.; Arora, T.; Lyu, L.; Stankevic, E.; Hansen, T.H.; Dechelotte, P.; et al. The gut microbiota contributes to the pathogenesis of anorexia nervosa in humans and mice. Nat. Microbiol. 2023, 8, 787–802.
- Singh, V.; Lee, G.; Son, H.; Koh, H.; Kim, E.S.; Unno, T.; Shin, J.H. Butyrate producers, “The Sentinel of Gut”: Their intestinal significance with and beyond butyrate, and prospective use as microbial therapeutics. Front. Microbiol. 2022, 13, 1103836.
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671.
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1286.
- Ferreira-Halder, C.V.; Faria, A.V.d.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648.
- Litvak, Y.; Byndloss, M.X.; Bäumler, A.J. Colonocyte metabolism shapes the gut microbiota. Science 2018, 362, eaat9076.
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277.
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194.
- Aizawa, E.; Tsuji, H.; Asahara, T.; Takahashi, T.; Teraishi, T.; Yoshida, S.; Ota, M.; Koga, N.; Hattori, K.; Kunugi, H. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J. Affect. Disord. 2016, 202, 254–257.
- Hanachi, M.; Manichanh, C.; Schoenenberger, A.; Pascal, V.; Levenez, F.; Cournede, N.; Dore, J.; Melchior, J.-C. Altered host-gut microbes symbiosis in severely malnourished anorexia nervosa (AN) patients undergoing enteral nutrition: An explicative factor of functional intestinal disorders? Clin. Nutr. 2019, 38, 2304–2310.
- Misra, M.; Klibanski, A. Endocrine consequences of anorexia nervosa. Lancet Diabetes Endocrinol. 2014, 2, 581–592.
- Scott, K.P.; Martin, J.C.; Campbell, G.; Mayer, C.-D.; Flint, H.J. Whole-Genome Transcription Profiling Reveals Genes Up-Regulated by Growth on Fucose in the Human Gut Bacterium “Roseburia inulinivorans”. J. Bacteriol. 2006, 188, 4340–4349.
- Pingitore, A.; Chambers, E.S.; Hill, T.; Maldonado, I.R.; Liu, B.; Bewick, G.; Morrison, D.J.; Preston, T.; Wallis, G.A.; Tedford, C.; et al. The diet-derived short chain fatty acid propionate improves beta-cell function in humans and stimulates insulin secretion from human islets in vitro. Diabetes Obes. Metab. 2017, 19, 257–265.
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015, 10, e0145274.
- Armougom, F.; Henry, M.; Vialettes, B.; Raccah, D.; Raoult, D. Monitoring bacterial community of human gut microbiota reveals an increase in Lactobacillus in obese patients and Methanogens in anorexic patients. PLoS ONE 2009, 4, e7125.
- Million, M.; Angelakis, E.; Maraninchi, M.; Henry, M.; Giorgi, R.; Valero, R.; Vialettes, B.; Raoult, D. Correlation between body mass index and gut concentrations of Lactobacillus reuteri, Bifidobacterium animalis, Methanobrevibacter smithii and Escherichia coli. Int. J. Obes. 2013, 37, 1460–1466.
- Ghenciulescu, A.; Park, R.J.; Burnet, P.W.J. The Gut Microbiome in Anorexia Nervosa: Friend or Foe? Front. Psychiatry 2020, 11, 611677.
- van de Pol, J.A.; van Best, N.; Mbakwa, C.A.; Thijs, C.; Savelkoul, P.H.; Arts, I.C.; Hornef, M.W.; Mommers, M.; Penders, J. Gut Colonization by Methanogenic Archaea Is Associated with Organic Dairy Consumption in Children. Front. Microbiol. 2017, 8, 355.
- Gabriel, T.; Massoubre, C.; Hanachi, M.; Doré, J.; Lambert, C.; Germain, N.; Galusca, B.; Paul, S. Association of gut-specific non-inflammatory T lymphocytes with chronic anorexia nervosa and constitutional thinness. Eur. Eat. Disord. Rev. 2023, 31, 76–86.
- Nilsson, I.A.K.; Millischer, V.; Göteson, A.; Hübel, C.; Thornton, L.M.; Bulik, C.M.; Schalling, M.; Landén, M. Aberrant inflammatory profile in acute but not recovered anorexia nervosa. Brain Behav. Immun. 2020, 88, 718–724.
- Dalton, B.; Bartholdy, S.; Robinson, L.; Solmi, M.; Ibrahim, M.A.A.; Breen, G.; Schmidt, U.; Himmerich, H. A meta-analysis of cytokine concentrations in eating disorders. J. Psychiatr. Res. 2018, 103, 252–264.
- Solmi, F.; Bulik, C.M.; De Stavola, B.L.; Dalman, C.; Khandaker, G.M.; Lewis, G. Longitudinal associations between circulating interleukin-6 and C-reactive protein in childhood, and eating disorders and disordered eating in adolescence. Brain Behav. Immun. 2020, 89, 491–500.
- Himmerich, H.; Bentley, J.; Kan, C.; Treasure, J. Genetic risk factors for eating disorders: An update and insights into pathophysiology. Ther. Adv. Psychopharmacol. 2019, 9, 2045125318814734.
- Breton, J.; Déchelotte, P.; Ribet, D. Intestinal microbiota and Anorexia Nervosa. Clin. Nutr. Exp. 2019, 28, 11–21.
- Fetissov, S.O.; Hökfelt, T. On the origin of eating disorders: Altered signaling between gut microbiota, adaptive immunity and the brain melanocortin system regulating feeding behavior. Curr. Opin. Pharmacol. 2019, 48, 82–91.
- Jumpertz, R.; Le, D.S.; Turnbaugh, P.J.; Trinidad, C.; Bogardus, C.; Gordon, J.I.; Krakoff, J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011, 94, 58–65.
More
Information
Subjects:
Psychology, Biological
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
412
Revisions:
2 times
(View History)
Update Date:
28 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




