Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Psychology, Biological
Anorexia nervosa (AN) remains a challenging condition in psychiatric management and its pathogenesis is not yet fully understood. An imbalance in the gut microbiota composition may contribute to its pathophysiology. There is an imbalance in gut microbiota composition leads to reduced short-chain fatty acids, contributing to a proinflammatory state in AN, which is also common in other psychiatric comorbidities. Microbial changes may also contribute to the semistarvation state through endocrine changes and altered energy utilization.
- anorexia nervosa
- gut microbiome
- microbiota
1. Introduction
Anorexia nervosa (AN) remains a challenging condition in psychiatric management, as the mortality rate is the highest among psychiatric disorders and can be relapsing or chronic in its course [9,10]. Less than half of the patients (46%) fully recover from AN, and it is commonly complicated by other psychiatric comorbidities such as affective disorders (1/4 AN patients) and anxiety disorders (1/4 AN patients) [10,11]. The highly comorbid nature of AN makes it difficult to discern if any gut microbiome changes are unique to AN alone or common in other psychiatric conditions. Hence, this study is limited in that it compares AN patients with patients with other psychiatric disorders, as well as healthy populations.
The pathogenesis is multifactorial and still not fully understood since it manifests in multiple non-CNS organ systems, such as immunological and endocrine dysfunction [12]. AN has a heritability component ranging from 28 to 74% and eight significant loci implicated in AN development have been identified [12,13]. Individual traits such as anxiety, perfectionism, and obsessive-compulsivity are both risk and prognostic factors [14].
AN patients typically have chronic caloric restriction, macronutrient and micronutrient deficiencies, changing food availability, and high fibre intake [15]. Genetics, infection, and inflammation can all directly affect dysbiosis, though profound changes in gut microbiota may be a result of changes in macronutrients [16]. The interlinking of multiple variables that affect dysbiosis highlights a limitation in investigating the role of dysbiosis in AN pathogenesis, whether it contributes to the maintenance or precedes the onset of AN.
AN patients are significantly underweight and require nutritional rehabilitation that comes with the potentially fatal risk of refeeding syndrome [12]. Current identification of high-risk refeeding syndrome patients follows guidelines developed by the National Institute for Health and Clinical Excellence (NICE) [17], and another screening test is the Short Nutritional Assessment Questionnaire [18]. However, these existing strategies to identify at-risk patients remain poorly validated, especially for predicting severe hypophosphatemia, a key characteristic of refeeding syndrome [19].
2. Gut Microbiome Changes in Anorexia Nervosa
2.1. Changes in Gut Microbiome Composition
2.1.1. Alpha Diversity
In microbiology, alpha diversity estimates the diversity within a single community, comprising the number of species present (richness) and the distribution of the number of organisms per species (evenness), i.e., their relative abundance and taxonomic distribution [40]. The diversity of a community is highly related to its environment and decreases in the setting of environmental changes (e.g., from a healthy to a diseased state). For example, alpha diversity is often decreased in irritable bowel syndrome [41]. In addition to observing decreased alpha diversity in AN, two studies observed an increase in alpha diversity in post-weight-restoration patients compared to before intervention [29,34], suggesting that low microbial diversity is implicated in lower BMI and greater starvation severity in AN. However, reduced alpha diversity is not a finding specific to AN, as it is also observed in AN patients with depression or anxiety. Depression is a prominent psychiatric feature secondary to the sequelae of semistarvation in AN [9], so it is possible that reduced alpha diversity may represent a more severe disease pathology [31]. However, there were inconsistencies in the alpha diversity changes in AN patients compared to HCs [27,32,37,38,39], and post- compared to pre-weight-restoration [31]. Differences in the size of patients’ stool samples and sample analysis techniques may explain these discrepancies. Furthermore, the discrepancy in alpha diversity data may be explained by the differences in the measurement indices (Shannon, Chao, Fischer, etc.)—there does not exist an absolute measure of diversity and each method has its own biases and advantages. Hence, the lack of specificity in choosing appropriate methods can lead to the oversimplification of diversity outcomes [42]. For example, the role of alpha diversity in general disease pathogenesis remains inconsistent, as studies in other disease states report an increase in alpha diversity [43,44].
Hence, it may be more useful to postulate the role of gut microbiota changes in AN pathogenesis by scrutinising individual taxa; however, this comes with another set of limitations. There is an inherent limitation in the interpretation of bacterial relative abundance for clinical analysis, as compositional data are not independent of each other and different biological scenarios can yield the same proportions of taxa over changes in time [45]. Reference frames have been identified as a way to alleviate false positives [45]. The reliability of compositional analysis is limited to the resolution of the sequencing method—16S rRNA sequencing or shotgun metagenomics and metagenomics relies on the known genomes of gut microorganisms [46].
2.1.2. Decreased Faecalibacterium prausnitzii
AN microbial communities were consistently depleted in Faecalibacterium [29,31,37,39] compared to HCs, and one study found a negative correlation between eating disorder scores and Faecalibacterium prausnitzii [28]. Faecalibacterium metabolise dietary fibres and other complex carbohydrates to produce short-chain fatty acids (SCFAs), such as butyrate [47]. SCFAs maintain the integrity of the intestinal barrier [48], promote immune cell recruitment to the gut, and increase the production of inflammatory mediators [49]. One type of SCFA is butyrate, and Faecalibacterium prausnitzii is the most common butyrate-producing species in faecal samples (5% abundance in healthy stool) [47]. Butyrate maintains the immunological aspect of gut barrier integrity by regulating Claudin-1 and synaptopodin expression, limiting pro-inflammatory cytokines (IL-6, IL-12), and inhibiting oncogenic pathways [47]. The synthesis of proinflammatory IL-6 and IL-12 is inhibited through the action of Faecalibacterium prausnitzii components inducing the IL-10 production in immune cells [50]. In addition to maintaining healthy intestinal barrier integrity, butyrate produced by Faecalibacterium prausnitzii also plays an important role in restricting the entry and establishment of pathogenic microbes [51]. Butyrate activates PPAR-ɣ signalling, which drives the high-oxygen-consuming metabolism within colonocytes, maintaining a state of epithelial hypoxia [51]. The anaerobic environment in the gut lumen, maintained by butyrate-producing bacteria, prevents colonisation by pathogenic Salmonella and E. coli [52]. Hence, in all these ways butyrate normally modulates the inflammatory responses within the gut. However, where these butyrate-producing species are decreased, authors hypothesize that there exists a pro-inflammatory state in the gut that contributes to AN (Figure 2).
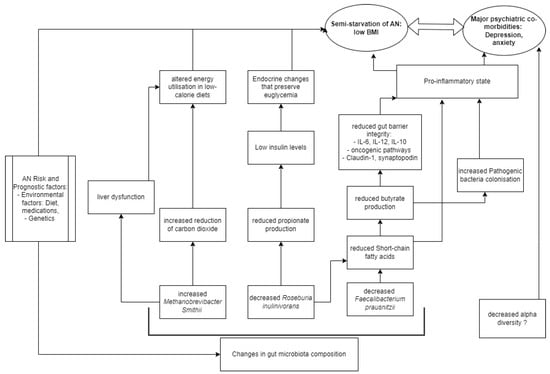
Figure 2. Model of AN pathogenesis related to gut microbiota.
There is a growing body of evidence to show that depression is associated with a chronic, low-grade inflammatory response and activation of cell-mediated immunity [53], similar to the speculation on the proinflammatory state in AN pathogenesis. The disrupted host inflammatory responses may be a result of butyrate or other SCFAs, as there is evidence to support that several SCFA-producing species (Bifidobacterium, Faecalibacterium, Lactobacillus) are reduced in depression and anxiety [53,54]. A negative correlation was observed between Faecalibacterium and the severity of depressive symptoms in a cross-sectional study [53]. Hence, a reduction in Faecalibacterium abundance is a finding non-specific to AN compared to other psychiatric disorders, but its abundance may negatively correlate to severe psychiatric disease [28].
2.1.3. Decreased Roseburia inulinivorans
Roseburia, another butyrate-producing Firmicute of similar significance, was consistently depleted [27,28,30,32], and negatively correlated with Eating Disorder Examination (17th edition) scores [34]. Levels of insulin are known to be reduced in AN patients compared to HCs, a phenomenon that helps preserve euglycemia by reducing cellular glucose uptake and glycogenesis [55], and the possible link to Roseburia species may be in propionate. Propionate, which is produced by Roseburia inulinivorans from fucose [56], has been shown to directly stimulate insulin secretion via protein kinase C and protect beta cells from apoptotic stimuli in the long term [57]. Decreased propionate levels in AN were observed in two studies [27,35], suggesting that the effective impacts of altered gut bacteria are endocrinological as well as neuro-inflammatory and immunological in contributing to the semistarvation state of AN (Figure 2).
2.1.4. Increased Methanobrevibacter smithii
Another consistent alteration in AN microbiome composition is the enrichment of Methanobrevibacter smithii [26,27,32,33], which is already well-documented as representing an adaptive response to prolonged caloric restriction [23]. Methanobrevibacter smithii uses hydrogen to reduce carbon dioxide to methane, allowing for optimal nutrient transformation in very low-calorie diets [26]. However, higher levels of the archaea have also been found in obesity, constipation, and irritable bowel syndrome [58], as well as non-alcoholic fatty liver disease and cirrhosis [27]. Borgo et al. also confirmed previously described increases in liver enzymes (ALT and AST) compared to HCs [27]. Hence, it is possible that Methanobrevibacter smithii contributes to altered metabolism, through a disruption of liver function commonly seen in AN patients (Figure 2). The contribution of Methanobrevibacter smithii to the semistarvation of AN is supported by Million et al., who observed a negative correlation between BMI and Methanobrevibacter smithii [33].
2.1.5. Comparison to Other Existing Pathophysiological Models
Gabriel et al. observed a chronic AN cohort (average illness duration 5.5 years) with a non-inflammatory cytokine profile; similarly, Nisson et al. studied a chronic AN cohort (average illness duration 10.8 years) with non-high levels of IL-6 in AN patients compared to HCs [59,60]. A meta-analysis of shorter-AN-duration cohorts showed elevated cytokine levels compared to HCs for IL-1beta, TNF-alpha, and IL-6; it appears that immune status varies according to AN disease duration [61,62]. This raises the limitation that most of the studies did not specify the time from the onset of disease, which means that the proinflammatory model in AN (Figure 2) is limited to the assumption of acute disease. In a review of genetic risk factors for eating disorders, Himmerich et al. highlighted an additional genetic role of SCFAs, as butyrate is an HDAC inhibitor with potential effects on gene expression in human cells [63].
Another hypothesis suggests that the energy needs of the gut microbiome may regulate the aberrant eating behaviour of individuals with AN; bacteria may produce modules that regulate the production of neurohormones involved in mood and eating behaviour or act directly as neurohormone-like molecules [64]. This describes a different link between SCFAs and AN, whereby SCFAs can directly act on enteroendocrine cells of the intestinal epithelium and activate the release of hormones contributing to satiation such as peptide tyrosine tyrosine or glucagon-like peptide 1 [64]. Researchers are increasingly focusing on the Enterobacteriaceae ClpB protein (caseinolytic peptidase B protein homologue) that can mimic the alpha-melanocyte-stimulating hormone involved in appetite control [64,65]. Three studies have found an increased prevalence of Clp-B-producing bacteria in AN patients [27,30,33].
2.2. Refeeding Syndrome
There have been numerous systematic reviews in recent years surrounding refeeding syndrome management in AN. Faecalibacterium, Roseburia and other butyrate-producing Firmicutes ferment dietary fibre and are consistently depleted in AN patients relative to healthy controls. Studies observed gut microbial composition post-weight-restoration, of which two are known to provide high-calorie diets (high in protein, fat, and predominantly carbohydrate) resulting in an increase in alpha diversity compared to before refeeding [29,34]. In contrast, only one study utilised a high-fibre, high-fat, and high-energy diet that resulted in decreased Bacteroidetes and increased Firmicutes compared to before refeeding [32]. The increase in Firmicutes is unlikely to be due to the high fibre consumption as total SCFA concentrations do not differ after refeeding; rather, it is likely attributable to the high fat or carbohydrate intake [71].
3. Conclusions
There is consistent evidence to support changes in gut microbiome composition in AN patients compared to health-weight controls, yet it remains unclear whether these changes contribute to the maintenance or precede the onset of AN. No results were obtained for refeeding syndrome. Microbial community changes in AN manifest in reduced anti-inflammatory effects at the level of the intestinal barrier, implicating a pro-inflammatory state in AN. However, this state is non-specific to AN compared to other psychiatric disorders.
This entry is adapted from the peer-reviewed paper 10.3390/pathophysiology31010006
This entry is offline, you can click here to edit this entry!
