
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Marvin Chávez-Sifontes | -- | 1183 | 2024-02-27 15:39:14 | | | |
| 2 | Wendy Huang | Meta information modification | 1183 | 2024-02-29 08:48:22 | | |
Video Upload Options
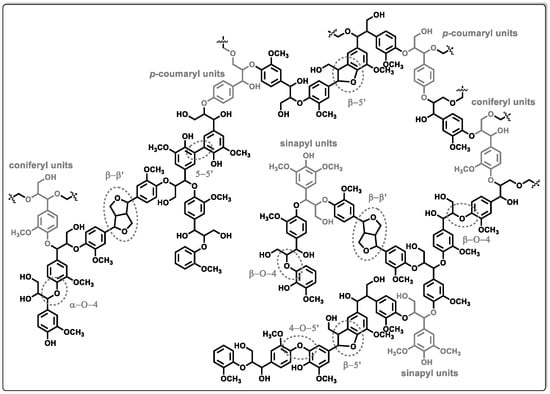
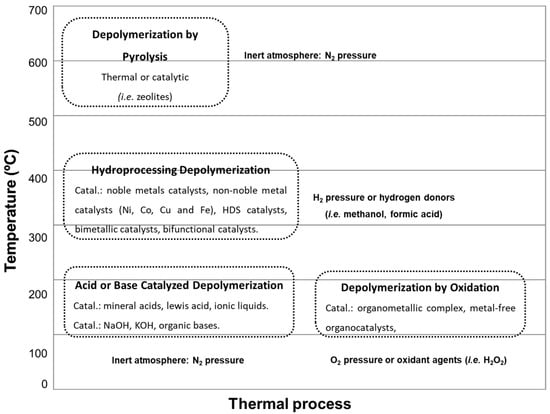
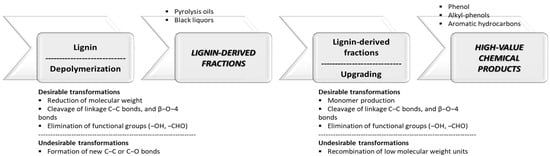
Lignocellulosic biomass is a raw material used for the production of bio-oils and black liquors. These biomass-derived fractions offer promising paths for the production of valuable chemical products. Various catalytic methods have been investigated for upgrading the biomass-derived fractions. Researchers are interested in the hydrodeoxygenation process (HDO); in this process, the oxygen groups are eliminated by breaking the C-O bonds and water as a product. Crucial factors influencing this optimization include temperature, hydrogen pressure, catalyst selection, and physicochemical attributes of the catalyst itself, such as the surface area, porosity, and acid–base properties. However, the intrinsic nature of lignin requires careful investigation. The chemical structural network of this biopolymer is significantly influenced by factors such as plant species and extraction process. Understanding and accounting for these variables are imperative for tailoring processes that efficiently harness the potential of lignin and its derivatives.
1. Introduction
2. Hydrodeoxygenation for Upgrading of Lignin-Derived Fractions
References
- Basbasan, A.J.; Hararak, B.; Winotapun, C.; Wanmolee, W.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Emerging Challenges on Viability and Commercialization of Lignin in Biobased Polymers for Food Packaging: A Review. Food Packag. Shelf Life 2022, 34, 100969.
- Tillou, J.G.; Ezeorah, C.J.; Kuchta, J.J.; Dissanayake Mudiyanselage, S.C.D.; Sitter, J.D.; Vannucci, A.K. A Review on Recent Trends in Selective Hydrodeoxygenation of Lignin Derived Molecules. RSC Sustain. 2023, 1, 1608–1633.
- Lang, M.; Li, H. Toward Mild Synthesis of Functional Chemicals from Lignin-Derived Phenolics via Emerging Catalytic Technology. Chem Catal. 2023, 3, 100609.
- Sun, P.; Wang, Z.; Li, C.; Tang, B.; Peng, C. Catalytic Conversion of Lignin and Its Derivatives to Alkanes over Multifunctional Catalysts: A Review. Fuel 2024, 361, 130726.
- Ročnik, T.; Likozar, B.; Jasiukaitytė-Grojzdek, E.; Grilc, M. Catalytic Lignin Valorisation by Depolymerisation, Hydrogenation, Demethylation and Hydrodeoxygenation: Mechanism, Chemical Reaction Kinetics and Transport Phenomena. Chem. Eng. J. 2022, 448, 137309.
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A Guide to Lignin Valorization in Biorefineries: Traditional, Recent, and Forthcoming Approaches to Convert Raw Lignocellulose into Valuable Materials and Chemicals. RSC Sustain. 2024, 2, 37–90.
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C.; et al. Kraft Lignin: A Valuable, Sustainable Resource, Opportunities and Challenges. ChemSusChem 2023, 16, e202300492.
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic Transformation of Lignin for the Production of Chemicals and Fuels. Chem. Rev. 2015, 115, 11559–11624.
- Chauhan, P.S.; Agrawal, R.; Satlewal, A.; Kumar, R.; Gupta, R.P.; Ramakumar, S.S.V. Next Generation Applications of Lignin Derived Commodity Products, Their Life Cycle, Techno-Economics and Societal Analysis. Int. J. Biol. Macromol. 2022, 197, 179–200.
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Nikkhah Dafchahi, M.; Acharya, B. Transforming Lignin into Renewable Fuels, Chemicals, and Materials: A Review. Bioresour. Technol. Reports 2023, 22, 101463–101487.
- Lu, X.; Gu, X. A Review on Lignin Pyrolysis: Pyrolytic Behavior, Mechanism, and Relevant Upgrading for Improving Process Efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106.
- Ventura, M.; Domine, M.E.; Chávez-Sifontes, M. Catalytic Processes for Lignin Valorization into Fuels and Chemicals (Aromatics). Curr. Catal. 2019, 8, 20–40.
- Zheng, J.L.; Wei, Q. Improving the Quality of Fast Pyrolysis Bio-Oil by Reduced Pressure Distillation. Biomass Bioenergy 2011, 35, 1804–1810.
- Figueirêdo, M.B.; Hita, I.; Deuss, P.J.; Venderbosch, R.H.; Heeres, H.J. Pyrolytic Lignin: A Promising Biorefinery Feedstock for the Production of Fuels and Valuable Chemicals. Green Chem. 2022, 24, 4680–4702.
- Korányi, T.I.; Fridrich, B.; Pineda, A.; Barta, K. Valorization of Lignocellulosic Biomass. ChemCatChem 2016, 8, 1422–1423.
- Phan, D.P.; Lee, E.Y. Controlled Hydrogenolysis over Heterogeneous Catalysts for Lignin Valorization. Catal. Rev. Sci. Eng. 2020, 62, 607–630.
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436.
- Yu, J.L.; Savage, P.E. Decomposition of Formic Acid under Hydrothermal Conditions. Ind. Eng. Chem. Res. 1998, 37, 2–10.
- Wang, X.; Zhang, Z.; Yan, Z.; Li, Q.; Zhang, Y. Catalysts with Metal-Acid Dual Sites for Selective Hydrodeoxygenation of Lignin Derivatives: Progress in Regulation Strategies and Applications. Appl. Catal. A Gen. 2023, 662, 119266.
- Kumar, A.; Jindal, M.; Maharana, S.; Thallada, B. Lignin Biorefinery: New Horizons in Catalytic Hydrodeoxygenation for the Production of Chemicals. Energy Fuels 2021, 35, 16965–16994.
- Prabhudesai, V.S.; Gurrala, L.; Vinu, R. Catalytic Hydrodeoxygenation of Lignin-Derived Oxygenates: Catalysis, Mechanism, and Effect of Process Conditions. Energy Fuels 2022, 36, 1155–1188.




