Lignocellulosic biomass is a raw material used for the production of bio-oils and black liquors. These biomass-derived fractions offer promising paths for the production of valuable chemical products. Various catalytic methods have been investigated for upgrading the biomass-derived fractions. Researchers are interested in the hydrodeoxygenation process (HDO); in this process, the oxygen groups are eliminated by breaking the C-O bonds and water as a product.
Crucial factors influencing this optimization include temperature, hydrogen pressure, catalyst selection, and physicochemical attributes of the catalyst itself, such as the surface area, porosity, and acid–base properties. However, the intrinsic nature of lignin requires careful investigation. The chemical structural network of this biopolymer is significantly influenced by factors such as plant species and extraction process. Understanding and accounting for these variables are imperative for tailoring processes that efficiently harness the potential of lignin and its derivatives.
1. Introduction
The exploration of renewable resources to produce fuels and chemicals has gained significant attention in response to escalating oil and petrochemical costs. Lignin, a biopolymer derived from lignocellulosic biomass, emerges as a prominent candidate in this field. Lignin considered a byproduct of industrial processes (i.e., paper manufacturing), has often been used in applications such as lignosulfonates, adhesive formulations, and polymer formulations [
1].
In recent years, lignin has gained increasing attention owing to its unique status as a renewable raw material with basic chemical aromatic structures. These chemical structures, which constitute fundamental building blocks, have the potential to serve as precursors for an array of aromatic chemical products, including phenols, alkyl phenols, BTX, cyclic alcohols, and cyclic hydrocarbons.
The interest in lignin is in line with the broader initiative to establish novel operational units within the biorefinery framework, marking a significant stride towards sustainable biomass utilization.
Several thermochemical and catalytic processes have been proposed to effectively depolymerize lignin and upgrade its derived fractions (i.e., black liquors), for example, hydrodeoxygenation (HDO). A paramount challenge lies in the meticulous optimization of the experimental conditions to maximize the yield of valuable liquid fractions.
Crucial factors influencing this optimization include temperature, hydrogen pressure, catalyst selection, and physicochemical attributes of the catalyst itself, such as the surface area, porosity, and acid–base properties. However, the intrinsic nature of lignin requires careful investigation. The chemical structural network of this biopolymer is significantly influenced by factors such as plant species and extraction process. Understanding and accounting for these variables are imperative for tailoring processes that efficiently harness the potential of lignin and its derivatives.
Numerous studies have focused on the hydrodeoxygenation of lignin model compounds, providing valuable insights into the influence of catalyst properties and experimental conditions, such as temperature and hydrogen pressure (H
2), on the reaction mechanism and catalytic performance. Those systematic studies have been presented in reviews, and that information has facilitated the development of catalysts with particular properties, for example, catalysts that exhibit enhanced resistance to aqueous medium, and medium with low pH values [
2,
3,
4].
However, the hydrodeoxygenation of lignin or lignin-derived fractions is a complex process encompassing intricate reactions, such as direct demethoxylation, hydrogenation, hydrogenolysis, demethylation, and trans-alkylation [
5]. A comprehensive understanding of the intricate interplay between the chemical structure of lignin and catalyst performance is imperative to selectively cleavage the bonds and obtain the desired monomeric products. Therefore, it is crucial to identify essential data such as the mass of lignin or lignin-derived fraction, the mass of the catalyst, the mass or volume of the solvent, the volume of the batch reactor, the hydrogen pressure (considering the temperature), and the mass or yield of products based on the mass of lignin. Those comprehensive data would allow the calculation of parameters such as O/C ratio, H/C ratio, and selectivity to monomers, facilitating performance comparisons across various research studies.
2. Hydrodeoxygenation for Upgrading of Lignin-Derived Fractions
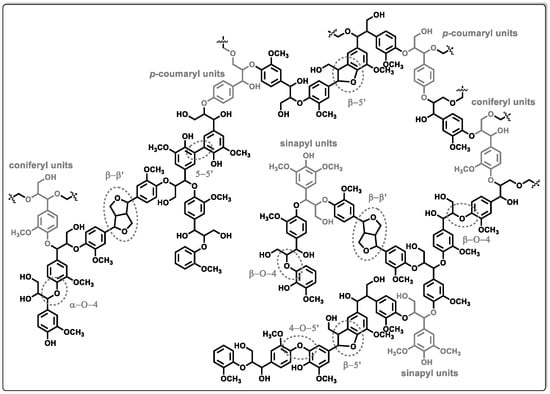
Lignin is a biopolymer structured by cross-linked phenolic co-polymers and comprises three phenyl-propane monomeric units:
p-coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol (see
Figure 1). Lignin is a renewable feedstock, ≈90% of the current lignin production is utilized as fuel for incineration in industrial plants for energy recovery [
6]. Approximately only a modest 10% of total lignin production is commercially transformed into industrial products, leaving the untapped potential for generating high-value chemical products [
7,
8].
Figure 1. Illustration of the chemical structure of lignin, highlighting the fundamental phenylpropanoid units (p-coumaryl, coniferyl, and sinapyl) and predominant linkages.
Recent attention has been focused on lignin applications and the processes for lignin conversion into biofuel and chemical product synthesis [
9,
10]. Biological and thermochemical processes (pyrolysis, depolymerization, and hydrogenolysis, see
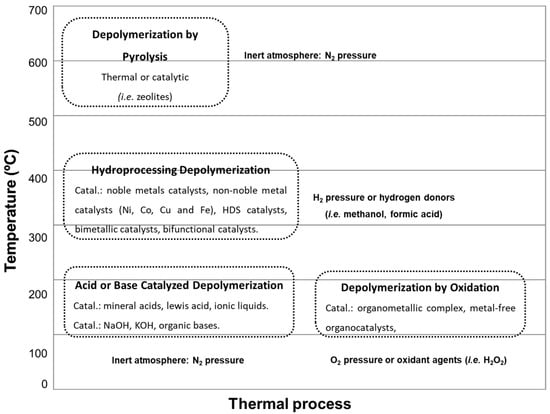
Figure 2) are the main processes used to transform lignin into useful chemical products.
Figure 2. Schematic overview of thermal processes and corresponding temperature ranges for the conversion of lignin into valuable chemical fractions.
Pyrolysis, a rapid heating process under an inert gas atmosphere at temperatures between 500 and 600 °C, produces reaction mixtures in different phases: liquid, solid, and gaseous. The process can be carried out under thermal or combined thermal/catalytic conditions [
11].
Depolymerization, generally carried out under pressure in an aqueous phase with a homogeneous alkaline catalyst at temperatures between 200 and 300 °C, produces a liquid organic phase containing oxygenated molecules (oligomers of medium molecular weight) and a solid fraction of residual lignin [
12]. There is also further research on other methods of lignin depolymerization to achieve organic fractions [
13].
The organic fraction (i.e., solid lignin or black liquor) derived from either pyrolysis or depolymerization is a mixture of various phenolic compounds that do not have optimal industrial properties. Therefore, subsequent processes are essential to improve properties such as water content, pH, O/C ratio, and H/C ratio [
14].
Currently, there are two extensively researched methods for upgrading lignin-derived fractions (lignin oils), zeolite cracking and hydrodeoxygenation. Zeolite cracking capitalizes on the high density of acid sites in zeolite materials, catalyzing cracking reactions (fractioning high molecular weight molecules into low molecular weight molecules) by eliminating oxygen, such as water, CO, and CO
2. The second method, hydrodeoxygenation, is conducted under hydrogen pressure, temperatures between 300 and 400 °C, and a catalyst with metallic function, primarily removing oxygen functional groups as water molecules [
15,
16].
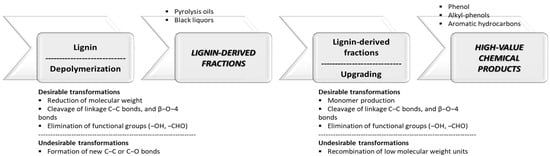
The transformation of lignin into industrial chemical products requires a combination of two stages: lignin depolymerization and hydrodeoxygenation of lignin-derived fractions (see
Figure 3). This process requires moderate or high temperatures and moderate or high hydrogen pressure, the hydrogen can be supplied externally or produced in situ from the decomposition of chemical donors such as formic acid or isopropyl alcohol [
17,
18]. The presence of a hydrogenating catalyst is crucial, and the process can be executed in aqueous or organic solvents.
Figure 3. The sequence of stages proposed for valorizing lignin into chemical products (first stage: depolymerization and second stage: upgrading). These two stages can be carried out in different reactors or the same reactor (one-pot).
Under these experimental conditions, a complex process, including the breaking of C−O structural bonds in lignin, occurs. This process comprises mechanisms such as hydrogenolysis, demethylation, and demethoxylation reactions, which are often improved by the incorporation of bifunctional catalysts (metal–acid properties) [
19]. The experimental setup allows for single (one-pot) or two-stage processes, yielding aromatic compounds with low oxygen content and aromatic hydrocarbons, including phenols, alkyl-phenols, and alkyl-benzenes [
20,
21].
This entry is adapted from the peer-reviewed paper 10.3390/catal14020146