
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | yangxiang wang | -- | 4119 | 2024-02-27 09:52:14 | | | |
| 2 | Wendy Huang | Meta information modification | 4119 | 2024-02-28 09:56:45 | | | | |
| 3 | Wendy Huang | Meta information modification | 4119 | 2024-03-04 06:56:41 | | |
Video Upload Options
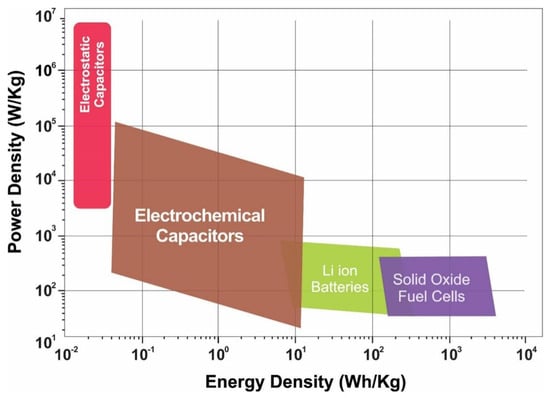
Supercapacitors (SCs) are a novel type of energy storage device that exhibit features such as a short charging time, a long service life, excellent temperature characteristics, energy saving, and environmental protection. The capacitance of SCs depends on the electrode materials. Currently, carbon-based materials, transition metal oxides/hydroxides, and conductive polymers are widely used as electrode materials. However, the low specific capacitance of carbon-based materials, high cost of transition metal oxides/hydroxides, and poor cycling performance of conductive polymers as electrodes limit their applications. Copper–sulfur compounds used as electrode materials exhibit excellent electrical conductivity, a wide voltage range, high specific capacitance, diverse structures, and abundant copper reserves, and have been widely studied in catalysis, sensors, supercapacitors, solar cells, and other fields.
1. Introduction
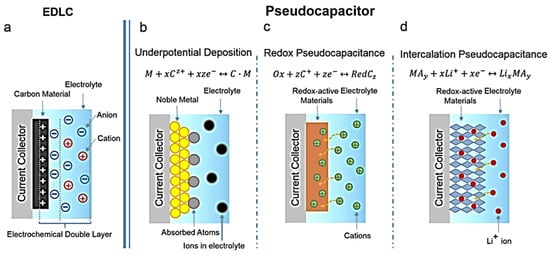
2. Copper–Sulfur Composite with Graphene for SC Applications
| NO. | Electrode Material |
Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance |
Retention Rate |
Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS/rGO | Three-electrode | −0.90~0.10 | 2 M KOH | 368.3 F g−1 (1 A g−1) | 88.4% after 1000 cycles | [17] |
| 2 | CuS/GO | Two-electrode | 0.00~1.00 | 3 M KOH | 197.45 F g−1 (5 mV s−1) | 90.35% after 1000 cycles | [18] |
| 3 | CuS/rGO | Three-electrode | 0.00~0.40 | 6 M KOH | 587.5 F g−1 (1 A g−1) | 95% after 2000 cycles | [19] |
| 4 | CuS/rGO | Three-electrode | 0.00~0.50 | 3 M KOH | 1604 F g−1 (2 A g−1) | 97% after 5000 cycles | [20] |
| 5 | Cu2S/rGO | Three-electrode | −1.00~0.00 | 1 M KOH | 1293 F g−1 (1 A g−1) | 94% after 10,000 cycles | [21] |
| 6 | CuS/rGO | Three-electrode | −1.10~−0.20 | 1 M LiClO4 | 1201.8 F g−1 (5 mV s−1) | 98% after 3000 cycles | [22] |
| 7 | CuS/rGO | Three-electrode | −0.20~0.40 | 6 M KOH | 2317.8 F g−1 (1 A g−1) | 96.2% after 1200 cycles | [23] |
| 8 | CuS@CQDs-GOH | Three-electrode | −0.10~0.50 | 6 M KOH | 920 F g−1 (1 A g−1) | 90% after 5000 cycles | [24] |
| 9 | CuS/GO | Three-electrode | 0.00~0.58 | 3 M KOH | 249 F g−1 (4 A g−1) | 95% after 5000 cycles | [25] |
| 10 | CuS/rGO | Three-electrode | 0.00~0.55 | 3 M KOH | 203 F g−1 (0.5 A g−1) | 90.8% after 10,000 cycles | [26] |
| 11 | CuS/CN | Three-electrode | −0.80~1.00 | 0.1 M Li2SO4 | 379 F g−1 (1 A g−1) | 72.46% after 500 cycles | [27] |
| 12 | CuS/GO | Three-electrode | −0.80~−0.15 | 6 M KOH | 497.8 F g−1 (0.2 A g−1) | 91.2% after 2000 cycles | [28] |
| 13 | CuS/rGO | Two-electrode | 0.00~1.00 | 6 M KOH | 906 F g−1 (1 A g−1) | 89% after 5000 cycles | [29] |
| 14 | CuS/rGO | Three-electrode | −0.20~0.60 | 2 M KOH | 1222.5 F g−1 (1 A g−1) | 91.2% after 2000 cycles | [30] |
| 15 | CuS/GO | Three-electrode | 0.00~0.60 | 3 M KOH | 250 F g−1 (0.5 A g−1) | 70% after 5000 cycles | [31] |
| 16 | CuS/rGO | Three-electrode | −1.00~0.00 | 2 M KOH | 3058 F g−1 (1 A g−1) | 60.3% after 1000 cycles | [32] |
| 17 | Cu2S/rGO | Three-electrode | −0.20~−0.45 | 3 M KOH | 1918.6 F g−1 (1 A g−1) | 95.4% after 5000 cycles | [33] |
3. Copper–Sulfur Composite with Carbon Nanotubes for SC Applications
| NO. | Electrode Material |
Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance |
Retention Rate |
Refs |
|---|---|---|---|---|---|---|---|
| 1 | CuS/CNTs | Three-electrode | 0.00~0.50 | 3 M KOH | 736.1 F g−1 (1 A g−l) | 92% after 5000 cycles | [34] |
| 2 | CuS/CNTs | Three-electrode | 0.00~0.60 | 6 M KOH | 467.02 F g−1 (0.5 A g−1) | 86% after 5000 cycles | [35] |
| 3 | CuS/CNT | Three-electrode | 0.00~0.50 | 2 M KOH | 122 F g−1 (1.2 A g−1) | 100% after 1000 cycles | [36] |
| 4 | CuS/CNTs | Three-electrode | −0.40~0.60 | 6 M KOH | 2831 F g−1 (1 A g−1) | 90% after 600 cycles | [37] |
| 5 | 3D-CuS/CNTs | Two-electrode | 0.00~0.60 | 2 M KOH | 2204 F g−1 (10 mA cm−2) | 89% after 10,000 cycles | [38] |
| 6 | CuS@CNT | Three-electrode | 0.00~1.00 | 2 M KOH | 1.51 F cm−2 (1.2 A g−1) | 92% after 1000 cycles | [39] |
| 7 | CuS/CNTs | Three-electrode | −0.20~0.60 | 2 M KOH | 566.4 F g−1 (1 A g−l) | 94.5% after 5000 cycles | [40] |
4. Copper Sulfide Composite with Activated Carbon in SC
5. Copper–Sulfur Compounds Compounded with CC in SC
6. Copper–Sulfur Composite with Acetylene Black in SC
7. MOF-Derived Copper–Sulfur Compound/Carbon-Based Nanocomposites for SC Applications
| NO. | Electrode Material |
Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance |
Retention Rate |
Refs |
|---|---|---|---|---|---|---|---|
| 1 | Cu1.96S/C | Two-electrode | 0.00~0.90 | 1 M KOH | 200 F g−1 (0.5 A g−1) | 80% after 3000 cycles | [51] |
| 2 | CuS/CNTs | Three-electrode | 0.00~0.50 | 6 M KOH | 606.7 F g−1 (1 A g−1) | 87.0% after 6000 cycles | [52] |
| 3 | Cu1.8S/C | Two-electrode | 1.00~3.00 | 1 M LiPF6 | 740 mAh g−1 (50 mA g−1) | 78% after 200 cycles | [53] |
| 4 | Carbon-coated Cu7S4 | Three-electrode | −0.20~0.70 | 1 M H2SO4 | 321.9 F g−1 (0.5 A g−1) | 78.1% after 3000 cycles | [54] |
| 5 | Cu9S8@C-CC@PPy | Three-electrode | −0.40~0.50 | 1 M KCl | 270.72 F g−1 (10 mV s−1) | 83.36% after 3000 cycles | [55] |
References
- Zhong, M.; Zhang, M.; Li, X. Carbon Nanomaterials and Their Composites for Supercapacitors. Carbon Energy 2022, 4, 950–985.
- Han, Z.; Fang, R.; Chu, D.; Wang, D.-W.; Ostrikov, K. Introduction to Supercapacitors. Nanoscale Adv. 2023, 5, 4015–4017.
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270.
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent Advances on Electrolyte Materials for SOFC: A Review. Inorg. Chem. Commun. 2023, 152, 110724.
- Samdhyan, K.; Chand, P.; Anand, H.; Saini, S. Development of Carbon-Based Copper Sulfide Nanocomposites for High Energy Supercapacitor Applications: A Comprehensive Review. J. Energy Storage 2022, 46, 103886.
- Volfkovich, Y.M. Self-Discharge of Supercapacitors: A Review. Russ. J. Electrochem. 2023, 59, 24–36.
- Anjana, P.M.; Sarath Kumar, S.R.; Rakhi, R.B. Direct Growth of Mn(OH)2/Co(OH)2 Nanocomposite on Carbon Cloth for Flexible Supercapacitor Electrodes. J. Energy Storage 2021, 33, 102151.
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854.
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643.
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and Confusions in Supercapacitor and Battery: Theory and Solutions. J. Power Sources 2018, 401, 213–223.
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280.
- Akinwolemiwa, B.; Wei, C.; Chen, G.Z. Mechanisms and Designs of Asymmetrical Electrochemical Capacitors. Electrochim. Acta 2017, 247, 344–357.
- Nagaraju, G.; Cha, S.M.; Sekhar, S.C.; Yu, J.S. Metallic Layered Polyester Fabric Enabled Nickel Selenide Nanostructures as Highly Conductive and Binderless Electrode with Superior Energy Storage Performance. Adv. Energy Mater. 2017, 7, 1601362.
- Shaikh, S.; Rabinal, M.K. Rapid Ambient Growth of Copper Sulfide Microstructures: Binder Free Electrodes for Supercapacitor. J. Energy Storage 2020, 28, 101288.
- Stevic, Z. Supercapacitors Based on Copper Sulfides. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2004.
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286.
- Xiao, W.; Zhou, W.; Feng, T.; Zhang, Y.; Liu, H.; Yu, H.; Tian, L.; Pu, Y. One-Pot Solvothermal Synthesis of Flower-like Copper Sulfide/Reduced Graphene Oxide Composite Superstructures as High-Performance Supercapacitor Electrode Materials. J. Mater. Sci. Mater. Electron. 2017, 28, 5931–5940.
- Balu, R.; Dakshanamoorthy, A. Synthesis of Wool Ball-like Copper Sulfide Nanospheres Embedded Graphene Nanocomposite as Electrode for High-performance Symmetric Supercapacitor Device. Int. J. Energy Res. 2022, 46, 6730–6744.
- El-Hout, S.I.; Mohamed, S.G.; Gaber, A.; Attia, S.Y.; Shawky, A.; El-Sheikh, S.M. High Electrochemical Performance of rGO Anchored CuS Nanospheres for Supercapacitor Applications. J. Energy Storage 2021, 34, 102001.
- BoopathiRaja, R.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. A Facile One Step Hydrothermal Induced Hexagonal Shaped CuS/rGO Nanocomposites for Asymmetric Supercapacitors. Mater. Today Proc. 2020, 26, 3507–3513.
- Bulakhe, R.N.; Alfantazi, A.; Rok Lee, Y.; Lee, M.; Shim, J.-J. Chemically Synthesized Copper Sulfide Nanoflakes on Reduced Graphene Oxide for Asymmetric Supercapacitors. J. Ind. Eng. Chem. 2021, 101, 423–429.
- Malavekar, D.B.; Lokhande, V.C.; Mane, V.J.; Kale, S.B.; Bulakhe, R.N.; Patil, U.M.; In, I.; Lokhande, C.D. Facile Synthesis of Layered Reduced Graphene Oxide–Copper Sulfide (rGO-CuS) Hybrid Electrode for All Solid-State Symmetric Supercapacitor. J. Solid State Electrochem. 2020, 24, 2963–2974.
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of Reduced Graphene Oxide Wrapped-Copper Sulfide Hollow Spheres as Electrode Material for Supercapacitor. Int. J. Hydrog. Energy 2015, 40, 10158–10167.
- De, B.; Kuila, T.; Kim, N.H.; Lee, J.H. Carbon Dot Stabilized Copper Sulphide Nanoparticles Decorated Graphene Oxide Hydrogel for High Performance Asymmetric Supercapacitor. Carbon 2017, 122, 247–257.
- Tian, Z.; Dou, H.; Zhang, B.; Fan, W.; Wang, X. Three-Dimensional Graphene Combined with Hierarchical CuS for the Design of Flexible Solid-State Supercapacitors. Electrochim. Acta 2017, 237, 109–118.
- Cui, Y.; Zhang, J.; Li, G.; Sun, Y.; Zhang, G.; Zheng, W. Ionic Liquid-Assisted Synthesis of rGO Wrapped Three-Dimensional CuS Ordered Nanoerythrocytes with Enhanced Performance for Asymmetric Supercapacitors. Chem. Eng. J. 2017, 325, 424–432.
- Chen, C.; Zhang, Q.; Ma, T.; Fan, W. Synthesis and Electrochemical Properties of Nitrogen-Doped Graphene/Copper Sulphide Nanocomposite for Supercapacitor. J. Nanosci. Nanotechnol. 2017, 17, 2811–2816.
- Li, X.; Zhou, K.; Zhou, J.; Shen, J.; Ye, M. CuS Nanoplatelets Arrays Grown on Graphene Nanosheets as Advanced Electrode Materials for Supercapacitor Applications. J. Mater. Sci. Technol. 2018, 34, 2342–2349.
- Zhao, T.; Yang, W.; Zhao, X.; Peng, X.; Hu, J.; Tang, C.; Li, T. Facile Preparation of Reduced Graphene Oxide/Copper Sulfide Composite as Electrode Materials for Supercapacitors with High Energy Density. Compos. Part B Eng. 2018, 150, 60–67.
- Zhu, W.; Ou, X.; Lu, Z.; Chen, K.; Ling, Y.; Zhang, H. Enhanced Performance of Hierarchical CuS Clusters Applying TRGO as Conductive Carrier for Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 5760–5770.
- Singhal, R.; Thorne, D.; LeMaire, P.K.; Martinez, X.; Zhao, C.; Gupta, R.K.; Uhl, D.; Scanley, E.; Broadbridge, C.C.; Sharma, R.K. Synthesis and Characterization of CuS, CuS/Graphene Oxide Nanocomposite for Supercapacitor Applications. AIP Adv. 2020, 10, 035307.
- Ghosh, K.; Srivastava, S.K. Enhanced Supercapacitor Performance and Electromagnetic Interference Shielding Effectiveness of CuS Quantum Dots Grown on Reduced Graphene Oxide Sheets. ACS Omega 2021, 6, 4582–4596.
- Zhuang, G.; Sun, Y.; Chen, X. CuS Cluster Microspheres Anchored on Reduced Graphene Oxide as Electrode Material for Asymmetric Supercapacitors with Outstanding Performance. J. Mater. Sci. Mater. Electron. 2021, 32, 4805–4814.
- Zhao, T.; Peng, X.; Zhao, X.; Hu, J.; Jiang, T.; Lu, X.; Zhang, H.; Li, T.; Ahmad, I. Preparation and Performance of Carbon Dot Decorated Copper Sulphide/Carbon Nanotubes Hybrid Composite as Supercapacitor Electrode Materials. J. Alloys Compd. 2020, 817, 153057.
- Quan, Y.; Zhang, M.; Wang, G.; Lu, L.; Wang, Z.; Xu, H.; Liu, S.; Min, Q. 3D Hierarchical Porous CuS Flower-Dispersed CNT Arrays on Nickel Foam as a Binder-Free Electrode for Supercapacitors. New J. Chem. 2019, 43, 10906–10914.
- Zhu, T.; Xia, B.; Zhou, L.; Lou, X.W.D. Arrays of Ultrafine CuS Nanoneedles Supported on a CNT Backbone for Application in Supercapacitors. J. Mater. Chem. 2012, 22, 7851.
- Huang, K.-J.; Zhang, J.-Z.; Xing, K. One-Step Synthesis of Layered CuS/Multi-Walled Carbon Nanotube Nanocomposites for Supercapacitor Electrode Material with Ultrahigh Specific Capacitance. Electrochim. Acta 2014, 149, 28–33.
- Lu, Y.; Liu, X.; Wang, W.; Cheng, J.; Yan, H.; Tang, C.; Kim, J.-K.; Luo, Y. Hierarchical, Porous CuS Microspheres Integrated with Carbon Nanotubes for High-Performance Supercapacitors. Sci. Rep. 2015, 5, 16584.
- Ravi, S.; Gopi, C.V.V.M.; Kim, H.J. Enhanced Electrochemical Capacitance of Polyimidazole Coated Covellite CuS Dispersed CNT Composite Materials for Application in Supercapacitors. Dalton Trans. 2016, 45, 12362–12371.
- Hou, X.; Liu, X.; Lu, Y.; Cheng, J.; Luo, R.; Yu, Q.; Wei, X.; Yan, H.; Ji, X.; Kim, J.-K.; et al. Copper Sulfide Nanoneedles on CNT Backbone Composite Electrodes for High-Performance Supercapacitors and Li-S Batteries. J. Solid State Electrochem. 2017, 21, 349–359.
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206.
- Li, C.; He, P.; Jia, L.; Zhang, X.; Zhang, T.; Dong, F.; He, M.; Wang, S.; Zhou, L.; Yang, T.; et al. Facile Synthesis of 3D CuS Micro-Flowers Grown on Porous Activated Carbon Derived from Pomelo Peel as Electrode for High-Performance Supercapacitors. Electrochim. Acta 2019, 299, 253–261.
- Wang, G.; Zhang, M.; Lu, L.; Xu, H.; Xiao, Z.; Liu, S.; Gao, S.; Yu, Z. One-Pot Synthesis of CuS Nanoflower-Decorated Active Carbon Layer for High-Performance Asymmetric Supercapacitors. ChemNanoMat 2018, 4, 964–971.
- Gong, S.-G.; Shi, Y.-H.; Su, Y.; Qi, F.; Song, Y.-H.; Yang, G.-D.; Li, B.; Wu, X.-L.; Zhang, J.-P.; Tong, C.-Y.; et al. Introduction of S-S Bond to Flexible Supercapacitors for High Mass Specific Capacity and Stability. J. Alloys Compd. 2022, 911, 165080.
- Zhou, W.; Miao, J.; Yan, X.; Li, Y.; Zhu, Y.; Zhang, W.; Zhang, M.; Zhu, W.; Javed, M.S.; Pan, J.; et al. Boosted Electrochemical Performance of CuS Anchored on Carbon Cloth as an Integrated Electrode for Quasi-Solid-State Flexible Supercapacitor. J. Electroanal. Chem. 2021, 897, 115610.
- Jin, K.; Zhou, M.; Zhao, H.; Zhai, S.; Ge, F.; Zhao, Y.; Cai, Z. Electrodeposited CuS Nanosheets on Carbonized Cotton Fabric as Flexible Supercapacitor Electrode for High Energy Storage. Electrochim. Acta 2019, 295, 668–676.
- Ahmad, W. P-Type NiO Nanoparticles Enhanced Acetylene Black as Efficient Counter Electrode for Dye-Sensitized Solar Cells. Mater. Res. Bull. 2015, 67, 185–190.
- Hou, H.; Yang, Y.; Zhu, Y.; Jing, M.; Pan, C.; Fang, L.; Song, W.; Yang, X.; Ji, X. An Electrochemical Study of Sb/Acetylene Black Composite as Anode for Sodium-Ion Batteries. Electrochim. Acta 2014, 146, 328–334.
- Sun, Y. Synthesis of a Ternary Polyaniline@acetylene Black-Sulfur Material by Continuous Two-Step Liquid Phase for Lithium Sulfur Batteries. Electrochim. Acta 2015, 158, 143–151.
- Huang, K.-J.; Zhang, J.-Z.; Jia, Y.-L.; Xing, K.; Liu, Y.-M. Acetylene Black Incorporated Layered Copper Sulfide Nanosheets for High-Performance Supercapacitor. J. Alloys Compd. 2015, 641, 119–126.
- Wu, R.; Wang, D.P.; Kumar, V.; Zhou, K.; Law, A.W.K.; Lee, P.S.; Lou, J.; Chen, Z. MOFs-Derived Copper Sulfides Embedded within Porous Carbon Octahedra for Electrochemical Capacitor Applications. Chem. Commun. 2015, 51, 3109–3112.
- Niu, H.; Liu, Y.; Mao, B.; Xin, N.; Jia, H.; Shi, W. In-Situ Embedding MOFs-Derived Copper Sulfide Polyhedrons in Carbon Nanotube Networks for Hybrid Supercapacitor with Superior Energy Density. Electrochim. Acta 2020, 329, 135130.
- Foley, S.; Geaney, H.; Bree, G.; Stokes, K.; Connolly, S.; Zaworotko, M.J.; Ryan, K.M. Copper Sulfide (CuxS) Nanowire-in-Carbon Composites Formed from Direct Sulfurization of the Metal-Organic Framework HKUST-1 and Their Use as Li-Ion Battery Cathodes. Adv. Funct. Mater. 2018, 28, 1800587.
- Li, L.; Liu, Y.; Han, Y.; Qi, X.; Li, X.; Fan, H.; Meng, L. Metal-Organic Framework-Derived Carbon Coated Copper Sulfide Nanocomposites as a Battery-Type Electrode for Electrochemical Capacitors. Mater. Lett. 2019, 236, 131–134.
- Liu, Y.-P.; Qi, X.-H.; Li, L.; Zhang, S.-H.; Bi, T. MOF-Derived PPy/Carbon-Coated Copper Sulfide Ceramic Nanocomposite as High-Performance Electrode for Supercapacitor. Ceram. Int. 2019, 45, 17216–17223.




