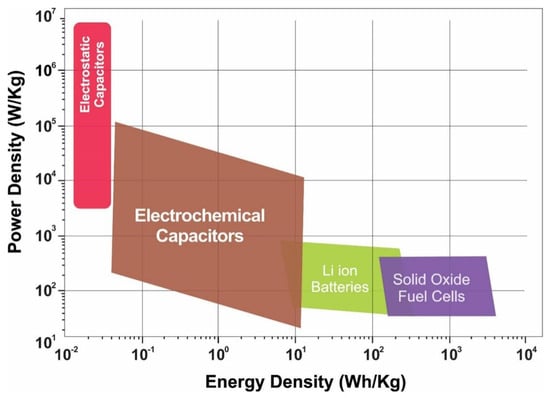
Supercapacitors (SCs) are a novel type of energy storage device that exhibit features such as a short charging time, a long service life, excellent temperature characteristics, energy saving, and environmental protection. The capacitance of SCs depends on the electrode materials. Currently, carbon-based materials, transition metal oxides/hydroxides, and conductive polymers are widely used as electrode materials. However, the low specific capacitance of carbon-based materials, high cost of transition metal oxides/hydroxides, and poor cycling performance of conductive polymers as electrodes limit their applications. Copper–sulfur compounds used as electrode materials exhibit excellent electrical conductivity, a wide voltage range, high specific capacitance, diverse structures, and abundant copper reserves, and have been widely studied in catalysis, sensors, supercapacitors, solar cells, and other fields.
- supercapacitors
- electrochemical properties
- specific capacitance
- stability
- copper–sulfur composite
- carbon-based materials
- graphene
- carbon nanotubes
- CC
- acetylene black (AB)
1. Introduction
2. Copper–Sulfur Composite with Graphene for SC Applications
| NO. | Electrode Material |
Measurement Type | MaterialOperating Window (V) | Electrolyte | Energy Storage | Measurement Type Performance |
Operating Window (V) | Electrolyte | Energy Storage PerformanceRetention Rate |
Retention Refs |
|---|---|---|---|---|---|---|---|---|---|---|
| Rate | Refs | |||||||||
| 1 | CuS/rGO | Three-electrode | −0.90~0.10 | |||||||
| 1 | Cu1.96S/C | Two-electrode | 2 M KOH | 0.00~0.90 | 1 M KOH368.3 F g−1 (1 A g | 200 F g−1−1) | 88.4% after 1000 cycles | [110][17] | ||
| 2 |
| NO. | Electrode Material |
Measurement Type | Operating Window (V) | Electrolyte | Energy Storage Performance |
Retention Rate |
Refs | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CuS/CNTs | Three-electrode | 0.00~0.50 | 3 M KOH | 736.1 F g−1 (1 A g−l) | (0.5 A g−192% after 5000 cycles | )[127][34] | 80% after 3000 cycles | [143][51] | |||||||||||
| CuS/GO | Two-electrode | 0.00~1.00 | 3 M KOH | 197.45 F g | ||||||||||||||||
| 2 | −1 | (5 mV s | CuS/CNTs | −1 | ) | 90.35% after 1000 cycles | Three-electrode | 0.00~0.60 | 6 M KOH | |||||||||||
| 2 | 467.02 F g | −1 | (0.5 A g | CuS/CNTs | −1 | Three-electrode | 0.00~0.50 | 6 M KOH) | 606.7 F g−1 (1 A g−1[111][18] | |||||||||||
| 86% after 5000 cycles | ) | [ | 128 | 87.0% after 6000 cycles | ] | [ | 35] | [144][52] | 3 | CuS/rGO | Three-electrode | 0.00~0.40 | 6 M KOH | |||||||
| 3 | Cu1.8 | 587.5 F g | −1 | (1 A g | −1) | 95% after 2000 cycles | [112 | |||||||||||||
| 3 | CuS/CNT | Three-electrode | ] | 0.00~0.50 | 2 M KOH | 122 F g−1 (1.2 A g−1) | 100% after 1000 cycles[19] | |||||||||||||
| S/C | [ | 129 | ] | [ | 36 | ] | Two-electrode | 1.00~3.00 | 1 M LiPF6 | 740 mAh g−1 (50 mA g−1) | 78% after 200 cycles | [23][53] | 4 | CuS/rGO | Three-electrode | 0.00~0.50 | 3 M KOH | 1604 F g−1 (2 A g−1) | 97% after 5000 cycles | [113][20] |
| 4 | CuS/CNTs | Three-electrode | −0.40~0.60 | 6 M KOH | ||||||||||||||||
| 4 | Carbon-coated Cu7S4 | 2831 F g | −1 | (1 A g | −1) | 90% after 600 cycles | [130][37] | Three-electrode | 5 | Cu2S/rGO | Three-electrode | −1.00~0.00 | 1 M KOH | 1293 F g−1 (1 A g−1) | 94% after 10,000 cycles | [114][21] | ||||
| −0.20~0.70 | 1 M H | 2 | SO | 4 | 321.9 F g−1 (0.5 A g−1) | 78.1% after 3000 cycles | [22][54] | 5 | 3D-CuS/CNTs | Two-electrode | 0.00~0.60 | 2 M KOH | 2204 F g−1 (10 mA cm−2) | 89% after 10,000 cycles | [131][38 | |||||
| 5 | Cu9S8@C-CC@PPy | ] | Three-electrode | −0.40~0.50 | 1 M KCl | 270.72 F g−1 (10 mV s−1) | 83.36% after 3000 cycles | [145][55] | 6 | CuS/rGO | Three-electrode | −1.10~−0.20 | 1 M LiClO4 | 1201.8 F g−1 (5 mV s−1) | 98% after 3000 cycles | |||||
| 6 | CuS@CNT | [ | Three-electrode | 0.00~1.00 | 2 M KOH | 1.51 F cm−2 (1.2 A g−1) | 115] | 92% after 1000 cycles[22] | ||||||||||||
| [ | 132 | ] | 7 | CuS/rGO | Three-electrode | −0.20~0.40 | 6 M KOH | 2317.8 F g−1 (1 A g−1) | 96.2% after 1200 cycles | [116][23] | ||||||||||
| [ | 39 | 8 | CuS@CQDs-GOH | Three-electrode | −0.10~0.50 | 6 M KOH | 920 F g−1 (1 A g−1) | 90% after 5000 cycles | [117][24] | |||||||||||
| 9 | CuS/GO | Three-electrode | 0.00~0.58 | 3 M KOH | 249 F g−1 (4 A g−1) | 95% after 5000 cycles | [118][25] | |||||||||||||
| 10 | CuS/rGO | Three-electrode | 0.00~0.55 | 3 M KOH | 203 F g−1 (0.5 A g−1) | 90.8% after 10,000 cycles | [119][26[28] | |||||||||||||
| ] | ||||||||||||||||||||
| 7 | CuS/CNTs | Three-electrode | −0.20~0.60 | 2 M KOH | 566.4 F g−1 (1 A g−l) | 94.5% after 5000 cycles | [133][40] | |||||||||||||
| ] | 11 | CuS/CN | Three-electrode | −0.80~1.00 | 0.1 M Li2SO4 | 379 F g−1 (1 A g−1) | 72.46% after 500 cycles | [120][27] | ||||||||||||
| 12 | CuS/GO | Three-electrode | −0.80~−0.15 | 6 M KOH | 497.8 F g−1 (0.2 A g−1) | 91.2% after 2000 cycles | [121] | 13 | CuS/rGO | Two-electrode | 0.00~1.00 | 6 M KOH | 906 F g−1 (1 A g−1) | 89% after 5000 cycles | [122][29] | |||||
| 14 | CuS/rGO | Three-electrode | −0.20~0.60 | 2 M KOH | 1222.5 F g−1 (1 A g−1) | 91.2% after 2000 cycles | [123][30] | |||||||||||||
| 15 | CuS/GO | Three-electrode | 0.00~0.60 | 3 M KOH | 250 F g−1 (0.5 A g−1) | 70% after 5000 cycles | [124][31] | |||||||||||||
| 16 | CuS/rGO | Three-electrode | −1.00~0.00 | 2 M KOH | 3058 F g−1 (1 A g−1) | 60.3% after 1000 cycles | [125][32] | |||||||||||||
| 17 | Cu2S/rGO | Three-electrode | −0.20~−0.45 | 3 M KOH | 1918.6 F g−1 (1 A g−1) | 95.4% after 5000 cycles | [126][33] |
3. Copper–Sulfur Composite with Carbon Nanotubes for SC Applications
Carbon nanotubes (CNTs) have a one-dimensional nanostructure, that is, a hexagonal network of tubular structures bonded by carbon material SP2. Because of their high electrical conductivity, excellent thermal and mechanical properties, light weight, large surface area, and unique pore structure, they can effectively improve the energy storage capacity and are widely applied for SC-active electrodes. Zhao et al. [127][34] prepared carbon dot (CQD)-modified CuS/CNTs composites with a three-dimensional grapevine-string-like structure by using the hydrothermal method at 180 °C for 12 h. The diameters of the CuS spheres were in the range 1–2 μm, and the doping of CQDs led to the reduction in the diameter of the CuS spheres to 100–500 nm, which caused the CuS spheres to interact closely with CNTs and accelerated the diffusion of electrons and ions. Their capacitance was 736.1 F g4. Copper Sulfide Composite with Activated Carbon in SC
Activated porous carbon (PPAC) has been widely used because of its excellent electrochemical properties, low cost, and large specific surface area [134][41]. Most of its micropores have diameters between 2 and 50 nm and surface areas up to 3000 m2 g−1. Li et al. [135][42] used a solvent-thermal method to homogeneously grow 3D CuS microflora consisting of stacked nanosheets on PPAC. The specific capacitance of the CuS/PPAC electrode was 954.0 F g−1 at a current density of 1.0 A g−1, higher than those of pure CuS (579.2 F g−1) and PPAC (329.6 F g−1). The energy density of CuS/PPAC was 47.70 Wh kg−1, which is nearly double that of CuS (29.08 Wh kg−1). The capacitance retention after 5000 charge/discharge cycles was 81.99%, which was higher than that of pure CuS-based electrodes (60.59%). Wang et al. [136][43] prepared porous CuS/AC with a three-dimensional hollow flower-like structure by using the solvent-thermal method. The specific surface area of the CuS/AC composite was as high as 539.34 m2 g−1 and the pore volume reached 0.22 cm3 g−1. The high specific surface area and mesoporous structure mitigated the capacity decay because of the volume change during charging and discharging and promoted the diffusion pathways for ionic conductivity and electrolyte penetration, as well as increased the active reaction sites between the electrolyte and electrodes. The introduction of the PPAC layer reduced the electrical resistance from 0.69 Ω for CuS to 0.31 Ω for CuS/AC, which improved the surface contact between CuS and the electrolyte and enhanced diffusion performance. The specific capacitance was 247 F g−1 at a current density of 0.5 A g−1, and the capacitance retention was 92% after 5000 cycles.5. Copper–Sulfur Compounds Compounded with CC in SC
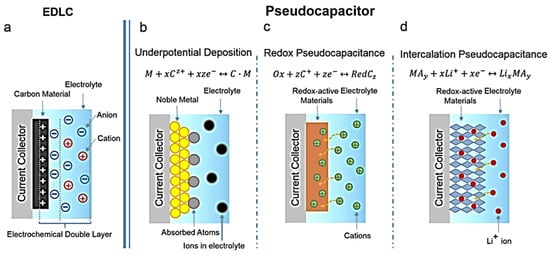
CC is a carbon-based material woven from carbon fibers that exhibits characteristics such as a light weight, low cost, high strength, low density, low thickness, and excellent flexibility. Gong et al. [137][44] grew CuS on CC in situ through chemical plating, achieving a specific capacity of 1387.1 F g−1 at a current density of 2 A g−1, and a capacity retention of 82.9% after 10,000 charge/discharge cycles. Zhou et al. [36][45] used the solvothermal method to grow dense CuS nanosheets on CC. The staggered ortho-hexagonal CuS nanosheets with suitable channels between them provided abundant electrochemically active sites and facilitated carrier exchange between the electrolyte and electrode interfaces. CuS generated after 4 h of the solvothermal reaction achieved the lowest impedance value (0.84 Ω) and a specific capacity of 436.5 mF cm−2 at a current density of 1 mA cm−2, which is higher than those of CC (2.36 mF cm−2), CuS/CC-3 (407.7 mF cm−2), and CuS/CC-5 (430.5 mF cm−2). The capacitance retention of the electrolyte after 5000 charge/discharge cycles was 75.1%. Jin et al. [138][46] deposited CuS nanosheets on conductive mesoporous CC through electrodeposition. The conductive CC served as both the current carrier and the skeleton of the composite. The g-CuS/CC and p-CuS/CC electrodes were prepared through the constant current and constant potential deposition methods, respectively, with g-CuS/CC achieving a specific surface area of 450.76 m2 g−1, which was larger than those of p-CuS/CC (397.84 m2 g−1) and CC (380.46 m2 g−1). At a current density of 2 mA cm−2, g-CuS/CC exhibited the capacitance of up to 4676 mF cm−2, higher than that of p-CuS/CC (3527 mF cm−2) and 10 times that of CC (490 mF cm−2), with a capacity retention of 89.8% after 10,000 cycles. The conductive CC acts as a skeleton framework for the electrodeposited composite as well as a collector for the electroactive material. This unique manufacturing process makes the interface extremely smooth while realizing electrochemical double-layer capacitor and pseudocapacitor energy storage, resulting in enhanced electrochemical performance.6. Copper–Sulfur Composite with Acetylene Black in SC
Acetylene black (AB) is a carbon material produced through the carbonization of acetylene by controlled combustion under pressure in air and has attracted much attention in the field of energy storage because of its light weight, low specific gravity, strong electrolyte absorption ability, chemical stability, low cost, and excellent electrical conductivity [139,140,141][47][48][49]. Huang et al. [142][50] used the solvothermal method to synthesize AB CuS nanosheet composite CuS/AB with a laminated structure. The intensity ratio of D-band to G-band ID/IG was 1.34, higher than that of pure AB (1.12), resulting in the generation of more defects and vacancies, an increase in the interfacial area between electrolyte/electrode, and enhanced electron transfer. The high conductance of AB and the short ion diffusion paths in the layered CuS nanosheets resulted in a CuS/AB specific capacitance of 2981 F g−1 at a current density of 1 A g−1, higher than those of pure CuS nanosheets (920 F g−1) and AB (658 F g−1), and the specific capacitance of the CuS/AB was considerably higher than that of pure AB (1.12). After 600 cycles, the capacity of CuS/AB was maintained at 92%, whereas the CuS and AB electrodes remained at 72.5% and 47.7%, respectively. The specific surface area of pure CuS nanosheets increased from 16.75 m2 g−1 to 62.37 m2 g−1, which increased the interface area between the electrolyte and electrode. Moreover, the high conductivity of AB and the CuS layer shortened the ion diffusion path and promoted electron transfer. AB anchored on CuS nanosheets to form a stable three-dimensional structure, reduces deformation, avoids the destruction of electrode materials, and maintains good stability during charge and discharge cycles.7. MOF-Derived Copper–Sulfur Compound/Carbon-Based Nanocomposites for SC Applications
Metal organic frameworks (MOFs) are three-dimensional inorganic/organic hybrid materials with a periodic network structure formed by the self-assembly of transition metal ions and organic ligands. Generally, with metal ions as the connecting point and organic ligands as a support, MOFs exhibit a large specific surface area, high porosity, low density, tailorability, tunable specific surface area, abundant pores, and structural diversity. MOFs are used as templates for special materials and precursors used to fabricate high porosity. The poor electrical conductivity and cyclic stability of MOF-derived porous materials make it difficult to obtain a high-performance SC when used as electrode materials. Therefore, combining the MOF-derived materials with other materials to form a composite electrode material for improving conductivity, specific capacity, and stability remains challenging. Cu-BTC[Cu3(C9H3O6)2(H2O)3]n, also known as HKUST-1, is a stable porous MOFs material. Wu et al. [143][51] used HKUST-1 as a template to prepare carbon-coated Cu1.96S in a single run by using the vulcanization method, which converted 10 nm ultrafine Cu1.96S nanoparticles uniformly embedded in octahedral porous carbon into porous Cu1.96S/C composites and maintained the octahedral morphology of MOFs during vulcanization and carbonization. Cu1.96S/C exhibits a large specific surface area (140.4 m2 g−1), which provides more active sites, and it has higher stability than Cu1.96S/C at a current density of 0.5 A g−1, a specific capacitance of 200 F g−1, and a capacity retention of 80% after 3000 constant current charge/discharge cycles. Niu et al. [144][52] prepared flexible composite electrodes, CuS/CNTs, by connecting HKUST-1-derived CuS polyhedra with CNTs. The uniformly distributed CuS polyhedra, consisting of a number of nanorods, had a large specific surface area of 126.4 m2 g−1 and excellent pore size distribution, resulting in a high specific capacity of 606.7 F g−1 at 1 A g−1, with the capacitance being 87.0% of the initial value after up to 6000 cycles at 5 A g−1. SC-related data on metal organic skeleton-derived carbon copper sulfide-based nanocomposites are listed in Table 3.| NO. | Electrode |
|---|
References
- Zhong, M.; Zhang, M.; Li, X. Carbon Nanomaterials and Their Composites for Supercapacitors. Carbon Energy 2022, 4, 950–985.
- Han, Z.; Fang, R.; Chu, D.; Wang, D.-W.; Ostrikov, K. Introduction to Supercapacitors. Nanoscale Adv. 2023, 5, 4015–4017.
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Chem. Rev. 2004, 104, 4245–4270.
- Vinchhi, P.; Khandla, M.; Chaudhary, K.; Pati, R. Recent Advances on Electrolyte Materials for SOFC: A Review. Inorg. Chem. Commun. 2023, 152, 110724.
- Samdhyan, K.; Chand, P.; Anand, H.; Saini, S. Development of Carbon-Based Copper Sulfide Nanocomposites for High Energy Supercapacitor Applications: A Comprehensive Review. J. Energy Storage 2022, 46, 103886.
- Volfkovich, Y.M. Self-Discharge of Supercapacitors: A Review. Russ. J. Electrochem. 2023, 59, 24–36.
- Anjana, P.M.; Sarath Kumar, S.R.; Rakhi, R.B. Direct Growth of Mn(OH)2/Co(OH)2 Nanocomposite on Carbon Cloth for Flexible Supercapacitor Electrodes. J. Energy Storage 2021, 33, 102151.
- Wang, F.; Wu, X.; Yuan, X.; Liu, Z.; Zhang, Y.; Fu, L.; Zhu, Y.; Zhou, Q.; Wu, Y.; Huang, W. Latest Advances in Supercapacitors: From New Electrode Materials to Novel Device Designs. Chem. Soc. Rev. 2017, 46, 6816–6854.
- Iro, Z.S.; Subramani, C.; Dash, S.S. A Brief Review on Electrode Materials for Supercapacitor. Int. J. Electrochem. Sci. 2016, 11, 10628–10643.
- Xie, J.; Yang, P.; Wang, Y.; Qi, T.; Lei, Y.; Li, C.M. Puzzles and Confusions in Supercapacitor and Battery: Theory and Solutions. J. Power Sources 2018, 401, 213–223.
- Shao, Y.; El-Kady, M.F.; Sun, J.; Li, Y.; Zhang, Q.; Zhu, M.; Wang, H.; Dunn, B.; Kaner, R.B. Design and Mechanisms of Asymmetric Supercapacitors. Chem. Rev. 2018, 118, 9233–9280.
- Akinwolemiwa, B.; Wei, C.; Chen, G.Z. Mechanisms and Designs of Asymmetrical Electrochemical Capacitors. Electrochim. Acta 2017, 247, 344–357.
- Nagaraju, G.; Cha, S.M.; Sekhar, S.C.; Yu, J.S. Metallic Layered Polyester Fabric Enabled Nickel Selenide Nanostructures as Highly Conductive and Binderless Electrode with Superior Energy Storage Performance. Adv. Energy Mater. 2017, 7, 1601362.
- Shaikh, S.; Rabinal, M.K. Rapid Ambient Growth of Copper Sulfide Microstructures: Binder Free Electrodes for Supercapacitor. J. Energy Storage 2020, 28, 101288.
- Stevic, Z. Supercapacitors Based on Copper Sulfides. Ph.D. Thesis, University of Belgrade, Belgrade, Serbia, 2004.
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286.
- Xiao, W.; Zhou, W.; Feng, T.; Zhang, Y.; Liu, H.; Yu, H.; Tian, L.; Pu, Y. One-Pot Solvothermal Synthesis of Flower-like Copper Sulfide/Reduced Graphene Oxide Composite Superstructures as High-Performance Supercapacitor Electrode Materials. J. Mater. Sci. Mater. Electron. 2017, 28, 5931–5940.
- Balu, R.; Dakshanamoorthy, A. Synthesis of Wool Ball-like Copper Sulfide Nanospheres Embedded Graphene Nanocomposite as Electrode for High-performance Symmetric Supercapacitor Device. Int. J. Energy Res. 2022, 46, 6730–6744.
- El-Hout, S.I.; Mohamed, S.G.; Gaber, A.; Attia, S.Y.; Shawky, A.; El-Sheikh, S.M. High Electrochemical Performance of rGO Anchored CuS Nanospheres for Supercapacitor Applications. J. Energy Storage 2021, 34, 102001.
- BoopathiRaja, R.; Parthibavarman, M.; Prabhu, S.; Ramesh, R. A Facile One Step Hydrothermal Induced Hexagonal Shaped CuS/rGO Nanocomposites for Asymmetric Supercapacitors. Mater. Today Proc. 2020, 26, 3507–3513.
- Bulakhe, R.N.; Alfantazi, A.; Rok Lee, Y.; Lee, M.; Shim, J.-J. Chemically Synthesized Copper Sulfide Nanoflakes on Reduced Graphene Oxide for Asymmetric Supercapacitors. J. Ind. Eng. Chem. 2021, 101, 423–429.
- Malavekar, D.B.; Lokhande, V.C.; Mane, V.J.; Kale, S.B.; Bulakhe, R.N.; Patil, U.M.; In, I.; Lokhande, C.D. Facile Synthesis of Layered Reduced Graphene Oxide–Copper Sulfide (rGO-CuS) Hybrid Electrode for All Solid-State Symmetric Supercapacitor. J. Solid State Electrochem. 2020, 24, 2963–2974.
- Huang, K.-J.; Zhang, J.-Z.; Liu, Y.; Liu, Y.-M. Synthesis of Reduced Graphene Oxide Wrapped-Copper Sulfide Hollow Spheres as Electrode Material for Supercapacitor. Int. J. Hydrog. Energy 2015, 40, 10158–10167.
- De, B.; Kuila, T.; Kim, N.H.; Lee, J.H. Carbon Dot Stabilized Copper Sulphide Nanoparticles Decorated Graphene Oxide Hydrogel for High Performance Asymmetric Supercapacitor. Carbon 2017, 122, 247–257.
- Tian, Z.; Dou, H.; Zhang, B.; Fan, W.; Wang, X. Three-Dimensional Graphene Combined with Hierarchical CuS for the Design of Flexible Solid-State Supercapacitors. Electrochim. Acta 2017, 237, 109–118.
- Cui, Y.; Zhang, J.; Li, G.; Sun, Y.; Zhang, G.; Zheng, W. Ionic Liquid-Assisted Synthesis of rGO Wrapped Three-Dimensional CuS Ordered Nanoerythrocytes with Enhanced Performance for Asymmetric Supercapacitors. Chem. Eng. J. 2017, 325, 424–432.
- Chen, C.; Zhang, Q.; Ma, T.; Fan, W. Synthesis and Electrochemical Properties of Nitrogen-Doped Graphene/Copper Sulphide Nanocomposite for Supercapacitor. J. Nanosci. Nanotechnol. 2017, 17, 2811–2816.
- Li, X.; Zhou, K.; Zhou, J.; Shen, J.; Ye, M. CuS Nanoplatelets Arrays Grown on Graphene Nanosheets as Advanced Electrode Materials for Supercapacitor Applications. J. Mater. Sci. Technol. 2018, 34, 2342–2349.
- Zhao, T.; Yang, W.; Zhao, X.; Peng, X.; Hu, J.; Tang, C.; Li, T. Facile Preparation of Reduced Graphene Oxide/Copper Sulfide Composite as Electrode Materials for Supercapacitors with High Energy Density. Compos. Part B Eng. 2018, 150, 60–67.
- Zhu, W.; Ou, X.; Lu, Z.; Chen, K.; Ling, Y.; Zhang, H. Enhanced Performance of Hierarchical CuS Clusters Applying TRGO as Conductive Carrier for Supercapacitors. J. Mater. Sci. Mater. Electron. 2019, 30, 5760–5770.
- Singhal, R.; Thorne, D.; LeMaire, P.K.; Martinez, X.; Zhao, C.; Gupta, R.K.; Uhl, D.; Scanley, E.; Broadbridge, C.C.; Sharma, R.K. Synthesis and Characterization of CuS, CuS/Graphene Oxide Nanocomposite for Supercapacitor Applications. AIP Adv. 2020, 10, 035307.
- Ghosh, K.; Srivastava, S.K. Enhanced Supercapacitor Performance and Electromagnetic Interference Shielding Effectiveness of CuS Quantum Dots Grown on Reduced Graphene Oxide Sheets. ACS Omega 2021, 6, 4582–4596.
- Zhuang, G.; Sun, Y.; Chen, X. CuS Cluster Microspheres Anchored on Reduced Graphene Oxide as Electrode Material for Asymmetric Supercapacitors with Outstanding Performance. J. Mater. Sci. Mater. Electron. 2021, 32, 4805–4814.
- Zhao, T.; Peng, X.; Zhao, X.; Hu, J.; Jiang, T.; Lu, X.; Zhang, H.; Li, T.; Ahmad, I. Preparation and Performance of Carbon Dot Decorated Copper Sulphide/Carbon Nanotubes Hybrid Composite as Supercapacitor Electrode Materials. J. Alloys Compd. 2020, 817, 153057.
- Quan, Y.; Zhang, M.; Wang, G.; Lu, L.; Wang, Z.; Xu, H.; Liu, S.; Min, Q. 3D Hierarchical Porous CuS Flower-Dispersed CNT Arrays on Nickel Foam as a Binder-Free Electrode for Supercapacitors. New J. Chem. 2019, 43, 10906–10914.
- Zhu, T.; Xia, B.; Zhou, L.; Lou, X.W.D. Arrays of Ultrafine CuS Nanoneedles Supported on a CNT Backbone for Application in Supercapacitors. J. Mater. Chem. 2012, 22, 7851.
- Huang, K.-J.; Zhang, J.-Z.; Xing, K. One-Step Synthesis of Layered CuS/Multi-Walled Carbon Nanotube Nanocomposites for Supercapacitor Electrode Material with Ultrahigh Specific Capacitance. Electrochim. Acta 2014, 149, 28–33.
- Lu, Y.; Liu, X.; Wang, W.; Cheng, J.; Yan, H.; Tang, C.; Kim, J.-K.; Luo, Y. Hierarchical, Porous CuS Microspheres Integrated with Carbon Nanotubes for High-Performance Supercapacitors. Sci. Rep. 2015, 5, 16584.
- Ravi, S.; Gopi, C.V.V.M.; Kim, H.J. Enhanced Electrochemical Capacitance of Polyimidazole Coated Covellite CuS Dispersed CNT Composite Materials for Application in Supercapacitors. Dalton Trans. 2016, 45, 12362–12371.
- Hou, X.; Liu, X.; Lu, Y.; Cheng, J.; Luo, R.; Yu, Q.; Wei, X.; Yan, H.; Ji, X.; Kim, J.-K.; et al. Copper Sulfide Nanoneedles on CNT Backbone Composite Electrodes for High-Performance Supercapacitors and Li-S Batteries. J. Solid State Electrochem. 2017, 21, 349–359.
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on Supercapacitors: Technologies and Materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206.
- Li, C.; He, P.; Jia, L.; Zhang, X.; Zhang, T.; Dong, F.; He, M.; Wang, S.; Zhou, L.; Yang, T.; et al. Facile Synthesis of 3D CuS Micro-Flowers Grown on Porous Activated Carbon Derived from Pomelo Peel as Electrode for High-Performance Supercapacitors. Electrochim. Acta 2019, 299, 253–261.
- Wang, G.; Zhang, M.; Lu, L.; Xu, H.; Xiao, Z.; Liu, S.; Gao, S.; Yu, Z. One-Pot Synthesis of CuS Nanoflower-Decorated Active Carbon Layer for High-Performance Asymmetric Supercapacitors. ChemNanoMat 2018, 4, 964–971.
- Gong, S.-G.; Shi, Y.-H.; Su, Y.; Qi, F.; Song, Y.-H.; Yang, G.-D.; Li, B.; Wu, X.-L.; Zhang, J.-P.; Tong, C.-Y.; et al. Introduction of S-S Bond to Flexible Supercapacitors for High Mass Specific Capacity and Stability. J. Alloys Compd. 2022, 911, 165080.
- Zhou, W.; Miao, J.; Yan, X.; Li, Y.; Zhu, Y.; Zhang, W.; Zhang, M.; Zhu, W.; Javed, M.S.; Pan, J.; et al. Boosted Electrochemical Performance of CuS Anchored on Carbon Cloth as an Integrated Electrode for Quasi-Solid-State Flexible Supercapacitor. J. Electroanal. Chem. 2021, 897, 115610.
- Jin, K.; Zhou, M.; Zhao, H.; Zhai, S.; Ge, F.; Zhao, Y.; Cai, Z. Electrodeposited CuS Nanosheets on Carbonized Cotton Fabric as Flexible Supercapacitor Electrode for High Energy Storage. Electrochim. Acta 2019, 295, 668–676.
- Ahmad, W. P-Type NiO Nanoparticles Enhanced Acetylene Black as Efficient Counter Electrode for Dye-Sensitized Solar Cells. Mater. Res. Bull. 2015, 67, 185–190.
- Hou, H.; Yang, Y.; Zhu, Y.; Jing, M.; Pan, C.; Fang, L.; Song, W.; Yang, X.; Ji, X. An Electrochemical Study of Sb/Acetylene Black Composite as Anode for Sodium-Ion Batteries. Electrochim. Acta 2014, 146, 328–334.
- Sun, Y. Synthesis of a Ternary Polyaniline@acetylene Black-Sulfur Material by Continuous Two-Step Liquid Phase for Lithium Sulfur Batteries. Electrochim. Acta 2015, 158, 143–151.
- Huang, K.-J.; Zhang, J.-Z.; Jia, Y.-L.; Xing, K.; Liu, Y.-M. Acetylene Black Incorporated Layered Copper Sulfide Nanosheets for High-Performance Supercapacitor. J. Alloys Compd. 2015, 641, 119–126.
- Wu, R.; Wang, D.P.; Kumar, V.; Zhou, K.; Law, A.W.K.; Lee, P.S.; Lou, J.; Chen, Z. MOFs-Derived Copper Sulfides Embedded within Porous Carbon Octahedra for Electrochemical Capacitor Applications. Chem. Commun. 2015, 51, 3109–3112.
- Niu, H.; Liu, Y.; Mao, B.; Xin, N.; Jia, H.; Shi, W. In-Situ Embedding MOFs-Derived Copper Sulfide Polyhedrons in Carbon Nanotube Networks for Hybrid Supercapacitor with Superior Energy Density. Electrochim. Acta 2020, 329, 135130.
- Foley, S.; Geaney, H.; Bree, G.; Stokes, K.; Connolly, S.; Zaworotko, M.J.; Ryan, K.M. Copper Sulfide (CuxS) Nanowire-in-Carbon Composites Formed from Direct Sulfurization of the Metal-Organic Framework HKUST-1 and Their Use as Li-Ion Battery Cathodes. Adv. Funct. Mater. 2018, 28, 1800587.
- Li, L.; Liu, Y.; Han, Y.; Qi, X.; Li, X.; Fan, H.; Meng, L. Metal-Organic Framework-Derived Carbon Coated Copper Sulfide Nanocomposites as a Battery-Type Electrode for Electrochemical Capacitors. Mater. Lett. 2019, 236, 131–134.
- Liu, Y.-P.; Qi, X.-H.; Li, L.; Zhang, S.-H.; Bi, T. MOF-Derived PPy/Carbon-Coated Copper Sulfide Ceramic Nanocomposite as High-Performance Electrode for Supercapacitor. Ceram. Int. 2019, 45, 17216–17223.
