Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Lazaros Tzelves | -- | 2643 | 2024-02-26 13:11:37 | | | |
| 2 | Camila Xu | Meta information modification | 2643 | 2024-02-27 02:16:56 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Feretzakis, G.; Juliebø-Jones, P.; Tsaturyan, A.; Sener, T.E.; Verykios, V.S.; Karapiperis, D.; Bellos, T.; Katsimperis, S.; Angelopoulos, P.; Varkarakis, I.; et al. AI and Radiomics in Bladder Cancer. Encyclopedia. Available online: https://encyclopedia.pub/entry/55460 (accessed on 07 February 2026).
Feretzakis G, Juliebø-Jones P, Tsaturyan A, Sener TE, Verykios VS, Karapiperis D, et al. AI and Radiomics in Bladder Cancer. Encyclopedia. Available at: https://encyclopedia.pub/entry/55460. Accessed February 07, 2026.
Feretzakis, Georgios, Patrick Juliebø-Jones, Arman Tsaturyan, Tarik Emre Sener, Vassilios S. Verykios, Dimitrios Karapiperis, Themistoklis Bellos, Stamatios Katsimperis, Panagiotis Angelopoulos, Ioannis Varkarakis, et al. "AI and Radiomics in Bladder Cancer" Encyclopedia, https://encyclopedia.pub/entry/55460 (accessed February 07, 2026).
Feretzakis, G., Juliebø-Jones, P., Tsaturyan, A., Sener, T.E., Verykios, V.S., Karapiperis, D., Bellos, T., Katsimperis, S., Angelopoulos, P., Varkarakis, I., Skolarikos, A., Somani, B., & Tzelves, L. (2024, February 26). AI and Radiomics in Bladder Cancer. In Encyclopedia. https://encyclopedia.pub/entry/55460
Feretzakis, Georgios, et al. "AI and Radiomics in Bladder Cancer." Encyclopedia. Web. 26 February, 2024.
Copy Citation
The advent of artificial intelligence (AI) and radiomics in oncology marks a pivotal moment in the evolution of personalized medicine, particularly in the diagnosis and treatment of urological cancers such as bladder, kidney, and prostate cancer. These cutting-edge technologies are reshaping the foundations of cancer care, heralding a paradigm shift towards precision-driven methods. The diagnosis and treatment of bladder cancer have been profoundly transformed by the recent advancements in AI and radiomics.
artificial intelligence
radiomics
urological cancers
oncology
bladder cancer
1. Introduction
The advent of artificial intelligence (AI) and radiomics in oncology marks a pivotal moment in the evolution of personalized medicine, particularly in the diagnosis and treatment of urological cancers such as bladder, kidney, and prostate cancer. These cutting-edge technologies are reshaping the foundations of cancer care, heralding a paradigm shift towards precision-driven methods. AI’s ability to process vast datasets rapidly is unveiling new prospects in the comprehension of complex cancer dynamics, allowing clinicians to extract meaningful insights for more accurate diagnoses and efficacious treatment strategies [1][2].
Despite these advancements, a gap remains in fully understanding the clinical implementation and integration of AI and radiomics in urological cancer care, necessitating a critical review of emerging trends and their tangible impacts on patient outcomes.
Based on a literature search (Figure 1), it can be observed that distinct trends in the scientific literature related to cancer research on PubMed from 2016 to 2023. Two lines illustrate the volume of published studies: one represents publications associated with “AI and Radiomics”, and the other depicts cancer research without these terms. The data indicate a significant upward trajectory in publications that involve AI and radiomics, reflecting the growing interest and integration of these technologies in cancer research. Conversely, publications without these keywords appear to remain relatively stable over the same period, suggesting a steady but less dramatic growth in general cancer research. This trend underlines the impact of technological advancements in the field of oncology, where AI and radiomics are becoming increasingly central to research, potentially driving innovation in the diagnosis, treatment, and prognosis of cancer. A notable example [1] is the use of a 3D deep radiomics pipeline in analyzing the CT scans of metastatic urothelial cancer patients. This approach differentiated between disease control and progression in response to immunotherapy, demonstrating AI’s potential as a non-invasive biomarker with a predictive accuracy of 82.5%. This underscores AI’s growing significance in personalized cancer care. A recent literature review [2] focuses on the integration of AI-powered radiomics in urologic oncology. It highlights significant advances in diagnostics and prognosis, like improved lesion detection in prostate cancer through machine learning (ML) and using radiomics for differentiating renal masses in kidney cancer. Challenges remain, such as small sample sizes and the need for broader validation.
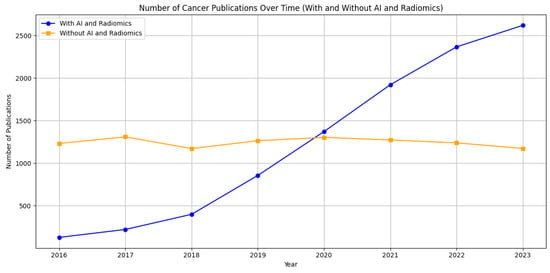
Figure 1. Comparative trends in cancer research publications from 2016 to 2023 on PubMed. The graph shows a marked increase in publications incorporating artificial intelligence and radiomics (blue line), contrasting with a relatively steady count of publications in broader cancer research (orange line). This illustrates the burgeoning role of advanced computational methods in cancer-related studies.
Radiomics enhances this landscape by offering an intricate analysis of medical images, identifying tumor patterns that escape the naked eye, thereby enriching the methodology in tumor characterization and affording a nuanced perspective of cancer behavior and progression [3]. Schawkat et al. took an in-depth look at novel imaging approaches for evaluating renal masses and renal cell carcinoma [3]. They discussed the updated Bosniak classification and the clear cell carcinoma likelihood score, along with newer modalities like contrast-enhanced ultrasound, dual-energy CT, and molecular imaging [3]. The integration of radiomics and artificial intelligence (AI) techniques was also explored. These contemporary diagnostic tools, combined with established methods, could address current limitations in renal mass characterization [3].
Evrimler et al. in their study assessed the potential of machine learning (ML)-based CT radiomics in predicting histological variants of bladder urothelial carcinoma, which is crucial for management [4]. It involved analyzing texture features from CT scans of 37 tumors, augmented by synthetic data. The study compared 15 ML algorithms, with the best models achieving high predictive accuracy [4].
In the realm of kidney cancer, particularly renal cell carcinoma (RCC), with its inherent heterogeneity, AI and radiomics adeptly address diagnostic and prognostic challenges. ML algorithms, when applied to MRI-derived radiomics features, have shown promise in distinguishing RCC subtypes and grades [5][6]. Cui et al. [5] investigated MR- and CT-based ML models for grading clear cell RCC. They included patients between 2009 and 2018 for model development and validation, with external validation from an independent institution and The Cancer Imaging Archive [5]. The study focused on the reproducibility and accuracy of texture features from MR and CT images, finding that MR- and CT-based models effectively distinguished high- from low-grade ccRCCs. Zhang et al. [6] focused on investigating radiomics features (RFs) related to the progression-free survival of RCC, aiming to develop a nomogram for individualized treatment reference. The research involved analyzing RFs and clinical data from 175 patients, using enhanced CT imaging and the LASSO algorithm for feature selection. The resulting radiomics nomogram integrated RFs and clinical predictors, demonstrating improved predictive accuracy for progression-free survival over clinical variables alone, highlighting its potential for personalized post-operative patient care in RCC [6]. The study’s radiomics nomogram, including age, clinical stage, Karnofsky performance status (KPS) score, and a weighted sum of six RFs, showed good discrimination and calibration. The C-index for the final model was 0.836 in the training set and 0.706 in the validation set, significantly outperforming the clinical-only model. The model’s clinical usefulness was confirmed through decision curve analysis, indicating its potential for guiding post-operative care in RCC patients. Similarly, predictive models derived from imaging data have been constructed to anticipate patient responses to treatments like immunotherapy and targeted agents, thus assisting in crafting personalized therapy plans [7].
Prostate cancer (PCa), being the most prevalent cancer in men, has witnessed significant advancements with the application of AI in MRI-based detection and management. Algorithms driven by AI have enhanced the precision in detecting clinically significant PCa, mitigating unnecessary biopsies, and facilitating informed treatment decisions [8][9]. Qiu et al. [8] developed a peritumoral radiomic-based ML model to differentiate between low and high Gleason-grade group lesions. They included 175 patients and used MRI sequences to delineate original and peritumoral regions of interest [8]. The model, which combined peritumoral features, outperformed other models with an area under the curve (AUC) of 0.850 and an average accuracy of 0.950, showing greater efficacy in predicting peripheral zone lesions. In a multi-institutional study [9], radiomic features from 3T mpMRI were analyzed to differentiate PCa detection in the transition and peripheral zones. The study extracted various features from MRI scans. Feature selection identified 10 distinct features for each zone. Zone-specific classifiers significantly improved cancer detection accuracy in the peripheral zone compared to a zone-ignorant classifier with an AUC of 0.61–0.71 across different institutions, highlighting the importance of considering zone differences in PCa diagnosis. Moreover, the amalgamation of AI with radiomics and genomics is carving out new paths in deciphering the molecular intricacies of PCa, potentially leading to more targeted and effective treatments [10][11]. In a study, authors [10] developed a deep learning (DL) algorithm to classify areas of increased uptake in bone scintigraphy scans, commonly used for screening metastatic bone disease (MBD). Trained and validated on scans from three European medical centers, the algorithm demonstrated high sensitivity and specificity (0.82 and 0.80, respectively) on an external test set. It significantly outperformed nuclear medicine physicians in terms of processing time [10].
In another study [11], ML models using radiomic features from mpMRI effectively detected and classified PCa in 191 patients who underwent mpMRI and biopsies. In reference to the study [11], Figure 2 exemplifies the meticulous process of image segmentation on mpMRI, which serves as a foundation for the machine learning models employed to detect and categorize prostate cancer. This figure (Figure 2) presents a representative case of a 60-year-old patient with a PSA level of 12.9 ng/mL and a non-suspicious digital rectal exam (DRE). The mpMRI images show the axial T2-weighted and ADC views where manual delineation has been carried out. The blue contour indicates the boundary of the entire prostate gland, capturing the complex anatomy inclusive of both the peripheral and transition zones. The green contour marks the transition zone specifically, while the red contour highlights the index lesion situated in the right peripheral zone at the apex, which was assigned a PI-RADS score of 4 due to its suspicious characteristics.
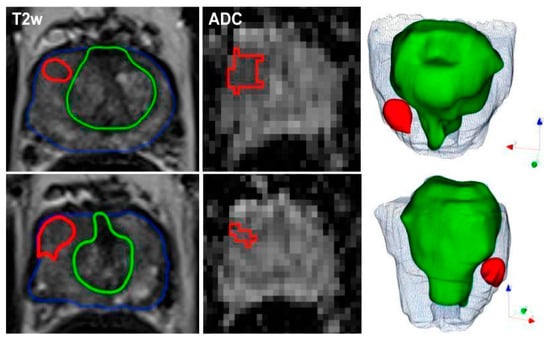
Figure 2. Representative segmentation of a patient on axial T2-weighted and ADC-images with an initial prostate-specific antigen level of 12.9 ng/ml, normal DRE result, and a highly suspicious lesion located medio-apically in the right peripheral zone (PI-RADS category 4). Targeted MRI/ultrasound-fusion biopsy confirmed the presence of PCa, Gleason 3+4, ISUP 2. The contours outline the following manual segmentations: blue—whole organ, green—transition zone, red—index lesion.
The precision of these delineations facilitated the extraction of radiomic features, which when combined with clinical assessments such as PI-RADS, PSAD, and DRE, yielded a robust model with high AUC values ranging from 0.844 to 0.889. This integrated approach not only improved the detection of malignant versus benign lesions but also proved effective in differentiating between clinically significant and insignificant prostate cancer. Such detailed segmentation underscores the combined model’s capability to exceed the diagnostic accuracy of the PI-RADS score alone and perform better than the mean ADC values in prognosticating clinically significant prostate cancer, as delineated in [11].
2. AI and Radiomics in Bladder Cancer
2.1. Recent Advancements
The diagnosis and treatment of bladder cancer have been profoundly transformed by the recent advancements in AI and radiomics. These innovative technologies provide a more nuanced understanding of bladder cancer, offering deeper insights into its characteristics and behavior through enhanced imaging analysis and predictive modeling. AI has revolutionized the diagnostic approach for bladder cancer, particularly when integrated with imaging modalities such as MRI and CT scans [12][13]. A study [12] utilizing a hybrid DL model showed impressive numerical results across three classification tasks: distinguishing normal tissue from bladder cancer, differentiating non-muscle-invasive bladder cancer (NMIBC) from muscle-invasive bladder cancer (MIBC), and identifying post-treatment changes (PTC) from MIBC. For the normal vs. bladder cancer task, the model achieved an accuracy of 86.07%, sensitivity of 96.75%, and specificity of 69.65% [12]. The best performance was observed with the LDA classifier on XceptionNet-based features [12]. In distinguishing NMIBC from MIBC, the accuracy was 79.72%, with a sensitivity of 66.62% and specificity of 87.39% [12]. For the post-treatment changes vs. MIBC task, the model recorded an accuracy of 74.96%, sensitivity of 80.51%, and specificity of 70.22% [12].
In an extensive analysis conducted by Sarkar et al., the complex stratification of bladder cancer stages was visually depicted through a series of axial CT scans with intravenous contrast. Figure 3 from Sarkar et al. presents a detailed view of the different stages, ranging from Ta, which represents non-invasive papillary carcinoma, to T4, indicative of advanced cancer with invasion beyond the bladder. The delineation of regions of interest (ROI) on the bladder wall corresponding to each stage emphasizes the potential challenges in visual diagnosis and the suitability of such images for advanced AI-based classification models [12]. As shown in Figure 3 above, the progression from non-muscle-invasive to muscle-invasive bladder cancer, through stages Ta, Tis, T1 (NMIBC) to T2, T3, and T4 (MIBC), underscores the necessity for precise staging, which is crucial for treatment planning and prognostic assessment.
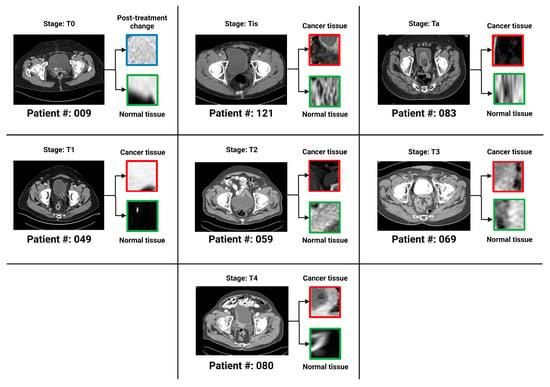
Figure 3. One bladder CT scan per stage (along with the corresponding regions of interest that were used in the various classification tasks) has been provided. The seven stages of urothelial carcinoma analyzed in the study are Ta, Tis, T0, T1, T2, T3 and T4 (T0 has not been shown in the figure because T0 represents a stage where the tissue of interest shows no evidence of malignancy).
Li et al. in their study [13] aimed to compare the effectiveness of radiomics, single-task DL, and multi-task DL methods in predicting MIBC using T2-weighted imaging (T2WI). It included 121 tumors, with 93 for training and 28 for testing. The AUC values in the training cohort were 0.920 (radiomics), 0.933 (single-task), 0.932 (multi-task), and 0.844, 0.884, and 0.932, respectively, in the test cohort [13]. The study concluded that all models showed good diagnostic performance, with the multi-task model being the most effective and focused on diseased tissue areas [13].
AI’s role in the realm of medical imaging marks a significant leap in oncology, especially in evaluating treatment responses. AI algorithms enable the quantification of radiologic characteristics beyond the capabilities of conventional imaging techniques, equipping clinicians with the ability to extract detailed quantitative data from images. This offers an unprecedented level of precision in diagnosing and monitoring diseases [1]. A notable study in this field utilized a 3D deep radiomics pipeline to analyze chest–abdomen CT scans, aiming to differentiate between disease control and progression in patients undergoing immunotherapy with immune checkpoint inhibitors (ICIs). This study involved monitoring 42 patients with metastatic urothelial cancer post-first-line platinum-based chemotherapy [1]. The deep learning pipeline, incorporating self-learned visual features and a deep self-attention mechanism, demonstrated a predictive accuracy of 82.5%, highlighting AI’s potential as a non-invasive biomarker for predicting disease control in response to ICIs in metastatic urothelial cancer [1].
Radiomics has revolutionized medical imaging, particularly in the analysis and interpretation of disease characteristics. By extracting an array of features from medical images, radiomics allows for an in-depth examination of disease manifestations. Processing these features through AI algorithms reveals complex patterns correlating with disease attributes and patient outcomes, often uncovering associations not apparent through conventional analysis [14]. In the context of bladder cancer (BCa), a significant study focused on constructing a CT-based deep learning radiomics nomogram (DLRN) to predict the pathological grade of BCa pre-operatively. The study, which extracted both handcrafted and DL radiomics features from multi-phase CT images of a large patient cohort, employed 11 machine learning classifiers [14]. The results showed the superiority of the support vector machine (SVM) classifier that combined these features, demonstrating the CT-based DLRN as a powerful diagnostic tool for differentiating between high and low-grade BCa, potentially guiding more precise pre-operative planning and personalized treatment approaches [14]. In another study [15], authors developed a CT-based radiomics model to preliminarily predict bladder-cancer grade. Patients with surgically resected bladder cancer, who had undergone CT urography, were divided into training and validation groups. The model used logistic regression and its performance was evaluated using the ROC curve, AUC, sensitivity, specificity, Positive Predictive Value (PPV), and Negative Predictive Value (NPV) [15]. An AUC of 0.950 in the training group and 0.860 in the validation group was detected. In the validation group, the model achieved 83.8% accuracy, 88.5% sensitivity, 72.7% specificity, 88.5% PPV, and 72.7% NPV, demonstrating its effectiveness.
2.2. Key Findings from Selected Studies
Key studies in this area include the use of CT-based deep-learning radiomics for creating a nomogram for the preoperative prediction of pathological grades in bladder cancer [16]. This research demonstrated the capability of radiomics features from pre-operative CT scans to construct a predictive model with remarkable accuracy, aiding clinicians in treatment planning and decision-making.
Rundo et al. in their study investigated the use of a 3-dimensional deep radiomics pipeline in analyzing CT scans for metastatic urothelial cancer patients [1]. This AI-based approach focused on differentiating disease control from progression in patients undergoing immunotherapy after first-line platinum-based chemotherapy failure [1]. The predictive accuracy of the pipeline was 82.5%, with a sensitivity of 96% and specificity of 60%, while including baseline clinical factors raised the accuracy to 92.5% [1]. This AI approach shows promise as a non-invasive biomarker for predicting responses to immunotherapy in metastatic urothelial cancer [1].
2.3. Implications for Diagnosis and Treatment
The advancements in AI and radiomics have substantial implications for bladder cancer diagnosis and treatment. By providing more accurate and comprehensive tumor information, these technologies can significantly refine the precision of diagnoses. This, in turn, facilitates more effective treatment strategies tailored to individual patient needs, particularly crucial in bladder cancer where treatment decisions heavily depend on the tumor’s stage and grade. As these technologies continue to evolve, their incorporation into clinical workflows is expected, heralding a sophisticated and patient-centric approach to bladder cancer management.
The predictive ability of AI and radiomics in forecasting tumor behavior and response to treatment is ushering in a new era of personalized medicine in bladder-cancer care. This promises more efficacious treatment plans with potentially fewer side effects and improved patient outcomes.
References
- Rundo, F.; Bersanelli, M.; Urzia, V.; Friedlaender, A.; Cantale, O.; Calcara, G.; Addeo, A.; Banna, G.L. Three-Dimensional Deep Noninvasive Radiomics for the Prediction of Disease Control in Patients with Metastatic Urothelial Carcinoma treated with Immunotherapy. Clin. Genitourin. Cancer 2021, 19, 396–404.
- Gelikman, D.G.; Rais-Bahrami, S.; Pinto, P.A.; Turkbey, B. AI-powered radiomics: Revolutionizing detection of urologic malignancies. Curr. Opin. Urol. 2024, 34, 1–7.
- Schawkat, K.; Krajewski, K.M. Insights into Renal Cell Carcinoma with Novel Imaging Approaches. Hematol. Oncol. Clin. N. Am. 2023, 37, 863–875.
- Evrimler, S.; Gedik, M.A.; Serel, T.A.; Ertunc, O.; Ozturk, S.A.; Soyupek, S. Bladder Urothelial Carcinoma: Machine Learning-based Computed Tomography Radiomics for Prediction of Histological Variant. Acad. Radiol. 2022, 29, 1682–1689.
- Cui, E.; Li, Z.; Ma, C.; Li, Q.; Lei, Y.; Lan, Y.; Yu, J.; Zhou, Z.; Li, R.; Long, W.; et al. Predicting the ISUP grade of clear cell renal cell carcinoma with multiparametric MR and multiphase CT radiomics. Eur. Radiol. 2020, 30, 2912–2921.
- Zhang, H.; Yin, F.; Chen, M.; Yang, L.; Qi, A.; Cui, W.; Yang, S.; Wen, G. Development and Validation of a CT-Based Radiomics Nomogram for Predicting Postoperative Progression-Free Survival in Stage I–III Renal Cell Carcinoma. Front. Oncol. 2022, 11, 742547.
- Nie, P.; Yang, G.; Wang, Y.; Xu, Y.; Yan, L.; Zhang, M.; Zhao, L.; Wang, N.; Zhao, X.; Li, X.; et al. A CT-based deep learning radiomics nomogram outperforms the existing prognostic models for outcome prediction in clear cell renal cell carcinoma: A multicenter study. Eur. Radiol. 2023, 33, 8858–8868.
- Qiu, Y.; Liu, Y.-F.; Shu, X.; Qiao, X.-F.; Ai, G.-Y.; He, X.-J. Peritumoral Radiomics Strategy Based on Ensemble Learning for the Prediction of Gleason Grade Group of Prostate Cancer. Acad. Radiol. 2023, 30 (Suppl. 1), S1–S13.
- Ginsburg, S.B.; Algohary, A.; Pahwa, S.; Gulani, V.; Ponsky, L.; Aronen, H.J.; Boström, P.J.; Böhm, M.; Haynes, A.; Brenner, P.; et al. Radiomic features for prostate cancer detection on MRI differ between the transition and peripheral zones: Preliminary findings from a multi-institutional study. J. Magn. Reson. Imaging 2017, 46, 184–193.
- Ibrahim, A.; Vaidyanathan, A.; Primakov, S.; Belmans, F.; Bottari, F.; Refaee, T.; Lovinfosse, P.; Jadoul, A.; Derwael, C.; Hertel, F.; et al. Deep learning based identification of bone scintigraphies containing metastatic bone disease foci. Cancer Imaging 2023, 23, 12.
- Woźnicki, P.; Westhoff, N.; Huber, T.; Riffel, P.; Froelich, M.F.; Gresser, E.; von Hardenberg, J.; Mühlberg, A.; Michel, M.S.; Schoenberg, S.O.; et al. Multiparametric MRI for Prostate Cancer Characterization: Combined Use of Radiomics Model with PI-RADS and Clinical Parameters. Cancers 2020, 12, 1767.
- Sarkar, S.; Min, K.; Ikram, W.; Tatton, R.W.; Riaz, I.B.; Silva, A.C.; Bryce, A.H.; Moore, C.; Ho, T.H.; Sonpavde, G.; et al. Performing Automatic Identification and Staging of Urothelial Carcinoma in Bladder Cancer Patients Using a Hybrid Deep-Machine Learning Approach. Cancers 2023, 15, 1673.
- Li, J.; Qiu, Z.; Cao, K.; Deng, L.; Zhang, W.; Xie, C.; Yang, S.; Yue, P.; Zhong, J.; Lyu, J.; et al. Predicting muscle invasion in bladder cancer based on MRI: A comparison of radiomics, and single-task and multi-task deep learning. Comput. Methods Programs Biomed. 2023, 233, 107466.
- Song, H.; Yang, S.; Yu, B.; Li, N.; Huang, Y.; Sun, R.; Wang, B.; Nie, P.; Hou, F.; Huang, C.; et al. CT-based deep learning radiomics nomogram for the prediction of pathological grade in bladder cancer: A multicenter study. Cancer Imaging 2023, 23, 89.
- Zhang, G.; Xu, L.; Zhao, L.; Mao, L.; Li, X.; Jin, Z.; Sun, H. CT-based radiomics to predict the pathological grade of bladder cancer. Eur. Radiol. 2020, 30, 6749–6756.
- Zhang, G.; Wu, Z.; Zhang, X.; Xu, L.; Mao, L.; Li, X.; Xiao, Y.; Ji, Z.; Sun, H.; Jin, Z. CT-based radiomics to predict muscle invasion in bladder cancer. Eur. Radiol. 2022, 32, 3260–3268.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
441
Revisions:
2 times
(View History)
Update Date:
27 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




