
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Raelene Pickering | -- | 2441 | 2024-02-26 06:19:55 | | | |
| 2 | Jessie Wu | -2 word(s) | 2439 | 2024-02-26 06:33:12 | | |
Video Upload Options
Melanocortins, a group of cleavage peptide products of pro-opiomelanocortin (POMC), activate melanocortin receptors on the surface of a diverse range of cell types, leading to different biological actions. They are so named because of their melanotropic activity, that is, the ability of melanocortins to increase pigmentation in melanocytes in the skin and hair follicles, increase concentrations of eumelanin and prevent an increase in photosensitive pheomelanin. Melanocortins are produced by POMC neurons in the pars intermedia of the pituitary gland, the hypothalamic arcuate nucleus and the dorsal medullary nucleus of the solitary tract. They can be distinguished by the presence of an invariant amino acid sequence in each melanocortin peptide, His-Phe-Arg-Trp. The melanocortins produced in humans include alpha-melanocyte stimulating hormone (α-MSH), beta-melanocyte stimulating hormone (β-MSH), gamma-melanocyte stimulating hormone (γ-MSH) and adrenocorticotropic hormone (ACTH).
1. Melanocortins and the Control of Neural Energy Homeostasis
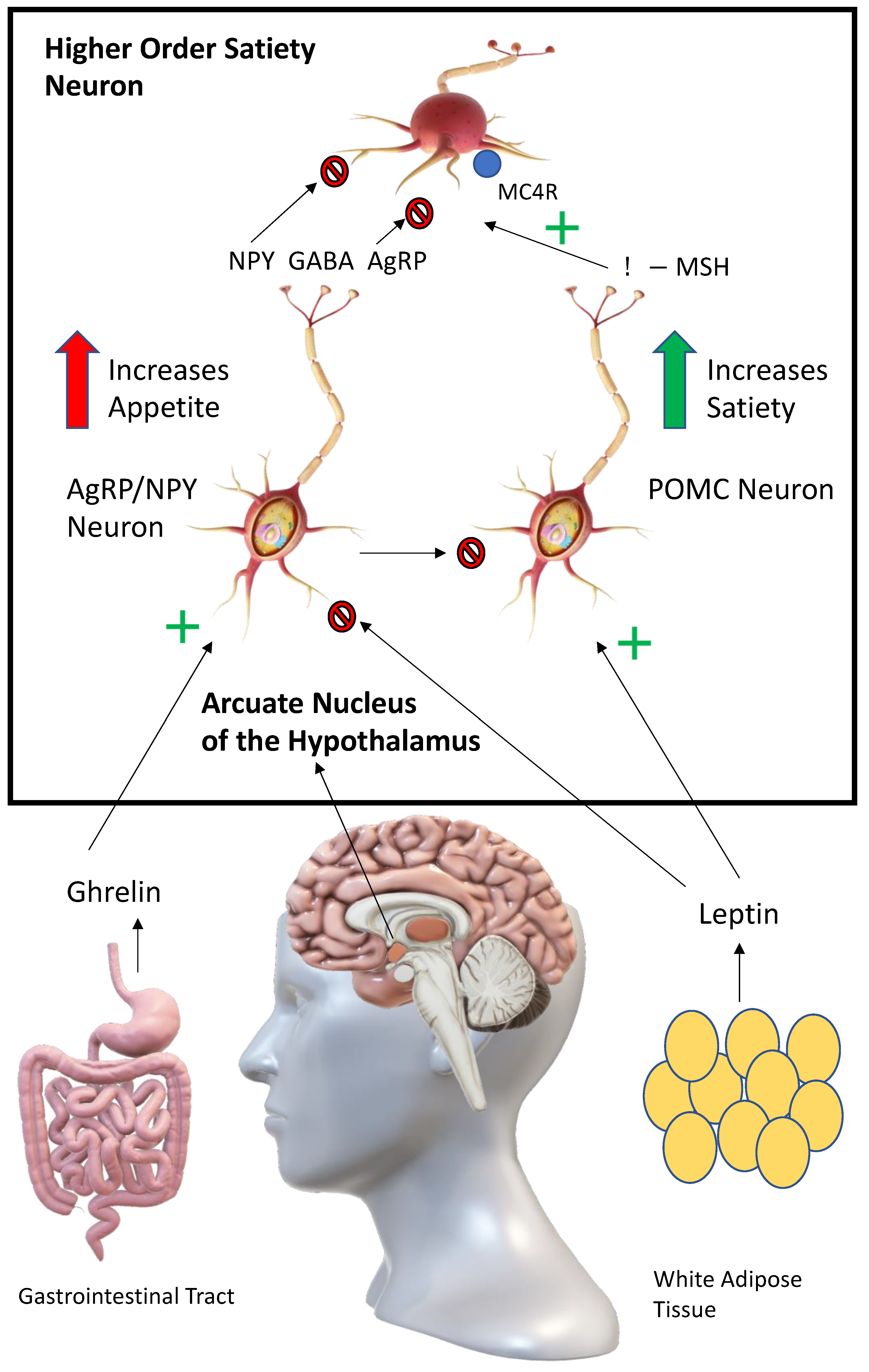
2. Melanocortins and Leptin: A Potential Downstream Advantage
3. An Additional Benefit: The Effects of Melanocortins in the Periphery
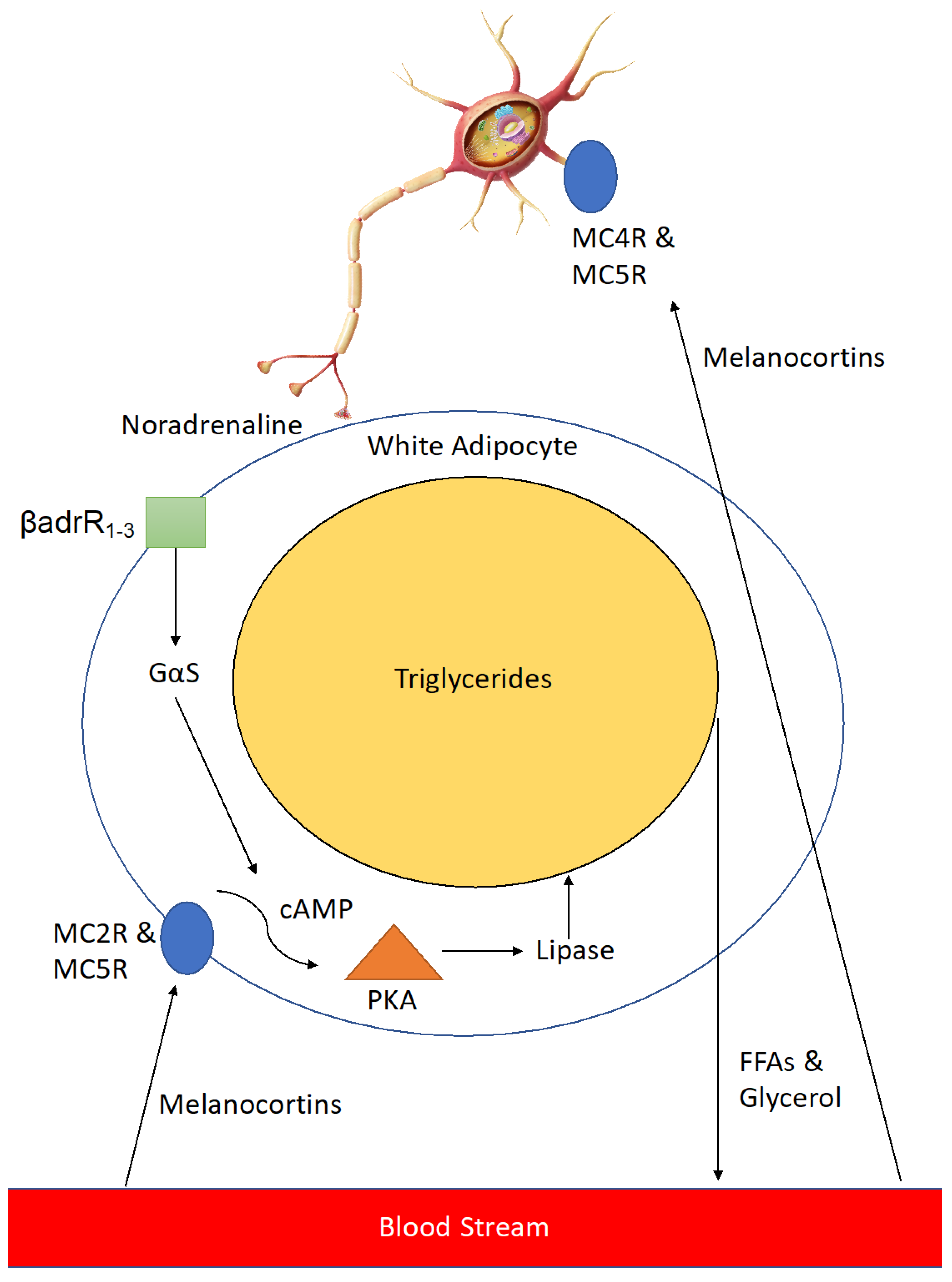
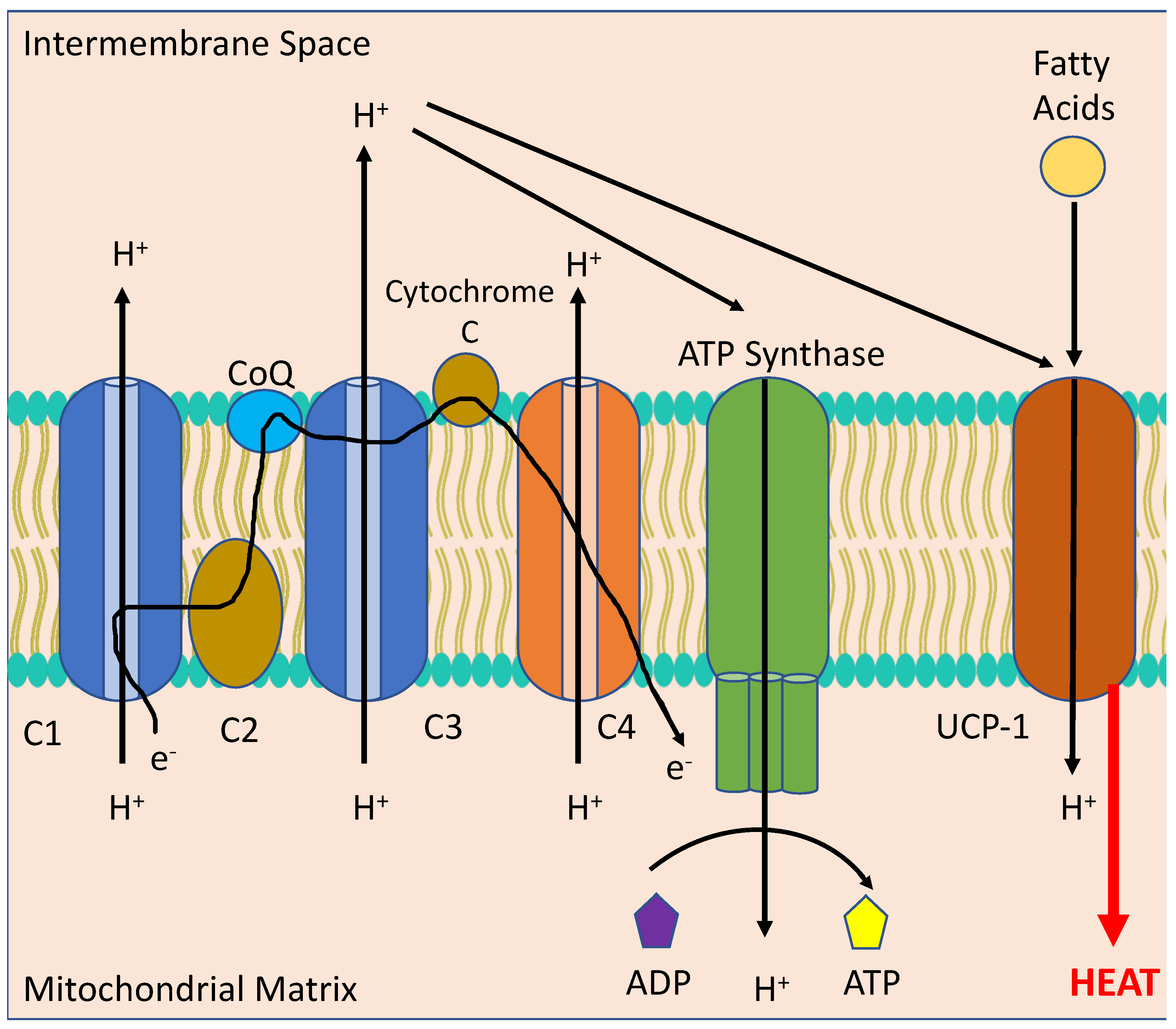
4. Setmelanotide: Emerging Evidence for the Use of a Clinical Melanocortin Analogue in Metabolic Disease
References
- Cui, H.; Sohn, J.W.; Gautron, L.; Funahashi, H.; Williams, K.W.; Elmquist, J.K.; Lutter, M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J. Comp. Neurol. 2012, 520, 4168–4183.
- Caron, A.; Dungan Lemko, H.M.; Castorena, C.M.; Fujikawa, T.; Lee, S.; Lord, C.C.; Ahmed, N.; Lee, C.E.; Holland, W.L.; Liu, C.; et al. POMC neurons expressing leptin receptors coordinate metabolic responses to fasting via suppression of leptin levels. eLife 2018, 7, e33710.
- Meneguetti, B.T.; Cardoso, M.H.; Ribeiro, C.F.A.; Felicio, M.R.; Pinto, I.B.; Santos, N.C.; Carvalho, C.M.E.; Franco, O.L. Neuropeptide receptors as potential pharmacological targets for obesity. Pharmacol. Ther. 2019, 196, 59–78.
- Zemel, M.B. Agouti/melanocortin interactions with leptin pathways in obesity. Nutr. Rev. 1998, 56, 271–274.
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318.
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432.
- Cummings, B.P. Leptin therapy in type 2 diabetes. Diabetes Obes. Metab. 2013, 15, 607–612.
- Biebermann, H.; Castañeda, T.R.; van Landeghem, F.; von Deimling, A.; Escher, F.; Brabant, G.; Hebebrand, J.; Hinney, A.; Tschöp, M.H.; Grüters, A.; et al. A role for beta-melanocyte-stimulating hormone in human body-weight regulation. Cell Metab. 2006, 3, 141–146.
- Collet, T.-H.; Dubern, B.; Mokrosinski, J.; Connors, H.; Keogh, J.; Oliveira, E.; Henning, E.; Poitou Bernert, C.; Oppert, J.-M.; Tounian, P.; et al. Evaluation of a Melanocortin-4 Receptor (MC4R) agonist (Setmelanotide) in MC4R deficiency. Mol. Metab. 2017, 6, 1321–1329.
- De Jonghe, B.C.; Hayes, M.R.; Zimmer, D.J.; Kanoski, S.E.; Grill, H.J.; Bence, K.K. Food intake reductions and increases in energetic responses by hindbrain leptin and melanotan II are enhanced in mice with POMC-specific PTP1B deficiency. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E644–E651.
- Eerola, K.; Virtanen, S.; Vähätalo, L.; Ailanen, L.; Cai, M.; Hruby, V.; Savontaus, M.; Savontaus, E. Hypothalamic γ-melanocyte stimulating hormone gene delivery reduces fat mass in male mice. J. Endocrinol. 2018, 239, 19–31.
- Kühnen, P.; Krude, H.; Biebermann, H. Melanocortin-4 Receptor Signalling: Importance for Weight Regulation and Obesity Treatment. Trends Mol. Med. 2019, 25, 136–148.
- Lee, Y.S.; Challis, B.G.; Thompson, D.A.; Yeo, G.S.; Keogh, J.M.; Madonna, M.E.; Wraight, V.; Sims, M.; Vatin, V.; Meyre, D.; et al. A POMC variant implicates beta-melanocyte-stimulating hormone in the control of human energy balance. Cell Metab. 2006, 3, 135–140.
- Backholer, K.; Smith, J.; Clarke, I.J. Melanocortins may stimulate reproduction by activating orexin neurons in the dorsomedial hypothalamus and kisspeptin neurons in the preoptic area of the ewe. Endocrinology 2009, 150, 5488–5497.
- Boghossian, S.; Park, M.; York, D.A. Melanocortin activity in the amygdala controls appetite for dietary fat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R385–R393.
- Campos, C.A.; Shiina, H.; Ritter, R.C. Central vagal afferent endings mediate reduction of food intake by melanocortin-3/4 receptor agonist. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 12636–12645.
- Chai, B.; Li, J.Y.; Zhang, W.; Wang, H.; Mulholland, M.W. Melanocortin-4 receptor activation inhibits c-Jun N-terminal kinase activity and promotes insulin signaling. Peptides 2009, 30, 1098–1104.
- Cote, I.; Green, S.M.; Morgan, D.; Carter, C.S.; Tumer, N.; Scarpace, P.J. Activation of the central melanocortin system in rats persistently reduces body and fat mass independently of caloric reduction. Can. J. Physiol. Pharmacol. 2018, 96, 308–312.
- Cote, I.; Sakarya, Y.; Kirichenko, N.; Morgan, D.; Carter, C.S.; Tumer, N.; Scarpace, P.J. Activation of the central melanocortin system chronically reduces body mass without the necessity of long-term caloric restriction. Can. J. Physiol. Pharmacol. 2017, 95, 206–214.
- Da Silva, A.A.; Freeman, J.N.; Hall, J.E.; do Carmo, J.M. Control of appetite, blood glucose, and blood pressure during melanocortin-4 receptor activation in normoglycemic and diabetic NPY-deficient mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R533–R539.
- Do Carmo, J.M.; da Silva, A.A.; Rushing, J.S.; Pace, B.; Hall, J.E. Differential control of metabolic and cardiovascular functions by melanocortin-4 receptors in proopiomelanocortin neurons. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R359–R368.
- Fani, L.; Bak, S.; Delhanty, P.; van Rossum, E.F.; van den Akker, E.L. The melanocortin-4 receptor as target for obesity treatment: A systematic review of emerging pharmacological therapeutic options. Int. J. Obes. 2014, 38, 163–169.
- Gavini, C.K.; Jones, W.C., 2nd; Novak, C.M. Ventromedial hypothalamic melanocortin receptor activation: Regulation of activity energy expenditure and skeletal muscle thermogenesis. J. Physiol. 2016, 594, 5285–5301.
- Monge-Roffarello, B.; Labbe, S.M.; Lenglos, C.; Caron, A.; Lanfray, D.; Samson, P.; Richard, D. The medial preoptic nucleus as a site of the thermogenic and metabolic actions of melanotan II in male rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R158–R166.
- Monge-Roffarello, B.; Labbe, S.M.; Roy, M.C.; Lemay, M.L.; Coneggo, E.; Samson, P.; Lanfray, D.; Richard, D. The PVH as a site of CB1-mediated stimulation of thermogenesis by MC4R agonism in male rats. Endocrinology 2014, 155, 3448–3458.
- Mul, J.D.; van Boxtel, R.; Bergen, D.J.; Brans, M.A.; Brakkee, J.H.; Toonen, P.W.; Garner, K.M.; Adan, R.A.; Cuppen, E. Melanocortin receptor 4 deficiency affects body weight regulation, grooming behavior, and substrate preference in the rat. Obesity 2012, 20, 612–621.
- Shrestha, Y.B.; Vaughan, C.H.; Smith, B.J., Jr.; Song, C.K.; Baro, D.J.; Bartness, T.J. Central melanocortin stimulation increases phosphorylated perilipin A and hormone-sensitive lipase in adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R140–R149.
- Song, C.K.; Vaughan, C.H.; Keen-Rhinehart, E.; Harris, R.B.; Richard, D.; Bartness, T.J. Melanocortin-4 receptor mRNA expressed in sympathetic outflow neurons to brown adipose tissue: Neuroanatomical and functional evidence. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R417–R428.
- Toda, C.; Shiuchi, T.; Lee, S.; Yamato-Esaki, M.; Fujino, Y.; Suzuki, A.; Okamoto, S.; Minokoshi, Y. Distinct effects of leptin and a melanocortin receptor agonist injected into medial hypothalamic nuclei on glucose uptake in peripheral tissues. Diabetes 2009, 58, 2757–2765.
- Wang, J.; Ling, S.; Usami, T.; Murata, T.; Narita, K.; Higuchi, T. Effects of ghrelin, corticotrophin-releasing hormone, and melanotan-II on food intake in rats with paraventricular nucleus lesions. Exp. Clin. Endocrinol. Diabetes Off. J. Ger. Soc. Endocrinol. Ger. Diabetes Assoc. 2007, 115, 669–673.
- Xu, Y.; Wu, Z.; Sun, H.; Zhu, Y.; Kim, E.R.; Lowell, B.B.; Arenkiel, B.R.; Xu, Y.; Tong, Q. Glutamate mediates the function of melanocortin receptor 4 on Sim1 neurons in body weight regulation. Cell Metab. 2013, 18, 860–870.
- Ye, Z.Y.; Li, D.P. Activation of the melanocortin-4 receptor causes enhanced excitation in presympathetic paraventricular neurons in obese Zucker rats. Regul. Pept. 2011, 166, 112–120.
- Zhang, Y.; Collazo, R.; Gao, Y.; Li, G.; Scarpace, P.J. Intermittent MTII application evokes repeated anorexia and robust fat and weight loss. Peptides 2010, 31, 639–643.
- Liu, Y.; Albrecht, E.; Schering, L.; Kuehn, C.; Yang, R.; Zhao, Z.; Maak, S. Agouti Signaling Protein and Its Receptors as Potential Molecular Markers for Intramuscular and Body Fat Deposition in Cattle. Front. Physiol. 2018, 9, 172.
- Grundlingh, J.; Dargan, P.I.; El-Zanfaly, M.; Wood, D.M. 2,4-dinitrophenol (DNP): A weight loss agent with significant acute toxicity and risk of death. J. Med. Toxicol. 2011, 7, 205–212.
- Colman, E. Dinitrophenol and obesity: An early twentieth-century regulatory dilemma. Regul. Toxicol. Pharmacol. 2007, 48, 115–117.
- Laiho, L.; Murray, J.F. The Multifaceted Melanocortin Receptors. Endocrinology 2022, 163, bqac083.
- Kühnen, P.; Clément, K.; Wiegand, S.; Blankenstein, O.; Gottesdiener, K.; Martini, L.L.; Mai, K.; Blume-Peytavi, U.; Grüters, A.; Krude, H. Proopiomelanocortin Deficiency Treated with a Melanocortin-4 Receptor Agonist. N. Engl. J. Med. 2016, 375, 240–246.
- Clément, K.; Biebermann, H.; Farooqi, I.S.; Van der Ploeg, L.; Wolters, B.; Poitou, C.; Puder, L.; Fiedorek, F.; Gottesdiener, K.; Kleinau, G.; et al. MC4R agonism promotes durable weight loss in patients with leptin receptor deficiency. Nat. Med. 2018, 24, 551–555.
- Haws, R.; Brady, S.; Davis, E.; Fletty, K.; Yuan, G.; Gordon, G.; Stewart, M.; Yanovski, J. Effect of setmelanotide, a melanocortin-4 receptor agonist, on obesity in Bardet-Biedl syndrome. Diabetes Obes. Metab. 2020, 22, 2133–2140.
- Forsythe, E.; Haws, R.M.; Argente, J.; Beales, P.; Martos-Moreno, G.; Dollfus, H.; Chirila, C.; Gnanasakthy, A.; Buckley, B.C.; Mallya, U.G.; et al. Quality of life improvements following one year of setmelanotide in children and adult patients with Bardet-Biedl syndrome: Phase 3 trial results. Orphanet J. Rare Dis. 2023, 18, 12.
- Haqq, A.M.; Chung, W.K.; Dollfus, H.; Haws, R.M.; Martos-Moreno, G.; Poitou, C.; Yanovski, J.A.; Mittleman, R.S.; Yuan, G.; Forsythe, E.; et al. Efficacy and safety of setmelanotide, a melanocortin-4 receptor agonist, in patients with Bardet-Biedl syndrome and Alström syndrome: A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial with an open-label period. Lancet Diabetes Endocrinol. 2022, 10, 859–868.
- Kühnen, P.; Wabitsch, M.; von Schnurbein, J.; Chirila, C.; Mallya, U.G.; Callahan, P.; Gnanasakthy, A.; Poitou, C.; Krabusch, P.M.; Stewart, M.; et al. Quality of life outcomes in two phase 3 trials of setmelanotide in patients with obesity due to LEPR or POMC deficiency. Orphanet J. Rare Dis. 2022, 17, 38.
- Fehm, H.L.; Smolnik, R.; Kern, W.; McGregor, G.P.; Bickel, U.; Born, J. The melanocortin melanocyte-stimulating hormone/adrenocorticotropin(4–10) decreases body fat in humans. J. Clin. Endocrinol. Metab. 2001, 86, 1144–1148.
- Spana, C.; Jordan, R.; Fischkoff, S. Effect of bremelanotide on body weight of obese women: Data from two phase 1 randomized controlled trials. Diabetes Obes. Metab. 2022, 24, 1084–1093.
- Chen, K.Y.; Muniyappa, R.; Abel, B.S.; Mullins, K.P.; Staker, P.; Brychta, R.J.; Zhao, X.; Ring, M.; Psota, T.L.; Cone, R.D.; et al. RM-493, a melanocortin-4 receptor (MC4R) agonist, increases resting energy expenditure in obese individuals. J. Clin. Endocrinol. Metab. 2015, 100, 1639–1645.
- Pierroz, D.D.; Ziotopoulou, M.; Ungsunan, L.; Moschos, S.; Flier, J.S.; Mantzoros, C.S. Effects of acute and chronic administration of the melanocortin agonist MTII in mice with diet-induced obesity. Diabetes 2002, 51, 1337–1345.
- Kievit, P.; Halem, H.; Marks, D.L.; Dong, J.Z.; Glavas, M.M.; Sinnayah, P.; Pranger, L.; Cowley, M.A.; Grove, K.L.; Culler, M.D. Chronic treatment with a melanocortin-4 receptor agonist causes weight loss, reduces insulin resistance, and improves cardiovascular function in diet-induced obese rhesus macaques. Diabetes 2013, 62, 490–497.
- Zhang, Y.; Matheny, M.; Tümer, N.; Scarpace, P.J. Aged-obese rats exhibit robust responses to a melanocortin agonist and antagonist despite leptin resistance. Neurobiol. Aging 2004, 25, 1349–1360.
- Blüher, S.; Ziotopoulou, M.; Bullen, J.W., Jr.; Moschos, S.J.; Ungsunan, L.; Kokkotou, E.; Maratos-Flier, E.; Mantzoros, C.S. Responsiveness to peripherally administered melanocortins in lean and obese mice. Diabetes 2004, 53, 82–90.
- Weiss, T.; Carr, R.D.; Pal, S.; Yang, L.; Sawhney, B.; Boggs, R.; Rajpathak, S.; Iglay, K. Real-World Adherence and Discontinuation of Glucagon-like Peptide-1 Receptor Agonists Therapy in Type 2 Diabetes Mellitus Patients in the United States. Patient Prefer. Adherence 2020, 14, 2337–2345.
- Mannucci, E.; Dicembrini, I.; Nreu, B.; Monami, M. Glucagon-like peptide-1 receptor agonists and cardiovascular outcomes in patients with and without prior cardiovascular events: An updated meta-analysis and subgroup analysis of randomized controlled trials. Diabetes Obes. Metab. 2019, 22, 203–211.
- Brown, E.; Cuthbertson, D.J.; Wilding, J.P. Newer GLP-1 receptor agonists and obesity-diabetes. Peptides 2018, 100, 61–67.
- Chang, M.; Chen, B.; Shaffner, J.; Dworkin, L.D.; Gong, R. Melanocortin System in Kidney Homeostasis and Disease: Novel Therapeutic Opportunities. Front. Physiol. 2021, 12, 651236.
- Langan, E.A.; Nie, Z.; Rhodes, L.E. Melanotropic peptides: More than just ‘Barbie drugs’ and ‘sun-tan jabs’? Br. J. Dermatol. 2010, 163, 451–455.




