Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Cheng Zhang | -- | 4070 | 2024-02-23 03:00:30 | | | |
| 2 | Lindsay Dong | -1 word(s) | 4069 | 2024-02-23 06:39:08 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Zhang, C.; An, G. Concentrated Solar Power Technology in Photocatalytic Water Purification. Encyclopedia. Available online: https://encyclopedia.pub/entry/55371 (accessed on 08 February 2026).
Zhang C, An G. Concentrated Solar Power Technology in Photocatalytic Water Purification. Encyclopedia. Available at: https://encyclopedia.pub/entry/55371. Accessed February 08, 2026.
Zhang, Cheng, Guangqi An. "Concentrated Solar Power Technology in Photocatalytic Water Purification" Encyclopedia, https://encyclopedia.pub/entry/55371 (accessed February 08, 2026).
Zhang, C., & An, G. (2024, February 23). Concentrated Solar Power Technology in Photocatalytic Water Purification. In Encyclopedia. https://encyclopedia.pub/entry/55371
Zhang, Cheng and Guangqi An. "Concentrated Solar Power Technology in Photocatalytic Water Purification." Encyclopedia. Web. 23 February, 2024.
Copy Citation
Photocatalysis, a promising semiconductor-based technology activated by free and eternal solar energy, has great potential for addressing environmental remediation and energy conversion challenges. Concentrated solar power (CSP) technologies, namely parabolic trough reflectors, solar power towers, parabolic dish reflectors and linear Fresnel reflectors, exhibited excellent feasibility for boosting solar-driven photocatalytic processes. Based on the structural characteristics of CSP technologies, the CSP-based photocatalytic reactors could be divided into concentrated types and non/low-concentrated types.
concentrated solar power (CSP) technology
photocatalysis
solar energy
1. Introduction
The sustainable development of the global economy and society is being disrupted by environmental deterioration, energy crises and other aspects [1]. The traditional way of production and life requires a large amount of non-renewable energy to drive, which not only further aggravates the problem of insufficient energy supply but also continues to destroy the ecological environment. Faced with this dilemma, the United Nations put forward the Sustainable Development Goals (SDGs), which emphasize the utilization of renewable energy and new production development modes [2]. Motivated by this, significant effort has been put into searching for new process strategies for energy conservation and emission reduction and studying their utilization patterns.
In the last few decades, photocatalysis has been intensively studied [3][4][5]. Under the illumination of incident light, the photocatalyst, as a semiconductor, could be excited to release electrons and holes, thereby leading to the subsequent generation of free radicals (such as superoxide radicals, hydroxyl radicals, etc.) with strong oxidation–reducing properties [6]. These free radicals could act on the structure of organic molecules and destroy their molecular bonds, resulting in the destruction and degradation of organic particles with no change occurring on the photocatalyst itself. Owing to the non-selectivity, cost-effectiveness, non-toxicity and eco-friendliness, photocatalytic technology exhibited impressive application potential in environment remediation (e.g., wastewater treatment [7][8], abatement of noxious gases [9], sterilization ([3][10], etc.), energy conversion (e.g., water splitting for hydrogen generation [11][12], hydrogen formation via ammonia decomposition [13][14], microalgae biorefinery [1], etc.) and so on. In order to boost this promising technology further into practical applications, the sources of energy used to drive photocatalytic reactions need to be carefully selected. Solar energy, as a natural source of light, has the following exciting characteristics: (1) permanent supply of energy, (2) wide distribution on earth, (3) easy utilization availability and (4) wide spectral band of wavelengths. Although the proportion of high-energy UV photons is only 3% to 5% of the total solar energy, it does not affect solar light to be the ideal driving force to motivate the photocatalytic processes [1][3][4][7].
As a bridge between sources of driving forces (solar energy) and users of driving forces (photocatalysis), photocatalytic reactors have been studied tirelessly in recent decades. The simplest photoreactor was a beaker placed under simulated solar light irradiation, which was filled with a photocatalyst and reactant [15][16]. Despite initiating the photocatalytic reaction, it was obviously far from practical application. Going one step further, some primitive but systematic prototype photoreactors have been developed and studied, including the classic inclined plate reactor (IPR) [7]. The structure of an IPR is characterized by an inclined plane facing the direction of solar light incidence, and the angle of inclination can be adjusted according to the local latitude. The photocatalyst is immobilized or flows down with wastewater in powder form, and the photocatalytic process is implemented upon the inclined plate. This simple and inexpensive design propelled IPR into widespread use, and an IPR-based wastewater treatment plant was even manufactured for purifying industrial wastewater from textile mills [17]. Nevertheless, one obvious disadvantage of IPR is that it leads to insufficient photocatalytic activity because of the low solar energy collecting efficiency brought on by passive acceptance of solar light [7]. Aiming to promote photocatalytic efficiency under actual sunlight, new photoreactor concepts are required. When it comes to effectively harvesting sunlight, it is natural to think of concentrated solar power (CSP) technology. CSP technology refers to the renewable energy project that concentrates, collects and converts solar energy into heat flux through a photo-thermal conversion process [18]. It has attracted great attention, owing to its unique advantages such as its superior capability for light and thermal collection, wide feasibility for multiple utilization purposes and high technical maturity. Generally, CSP technology can be divided into parabolic trough reflectors (PTRs), solar power towers (SPTs), parabolic dish reflectors (PDRs) and linear Fresnel reflectors (LFRs) according to their system configurations [19]. Since the effective solar energy collection capability of CSP technology matches the demand for solar energy-driven photocatalytic processes, it is not surprising that CSP-based photoreactors are widely developed and studied.
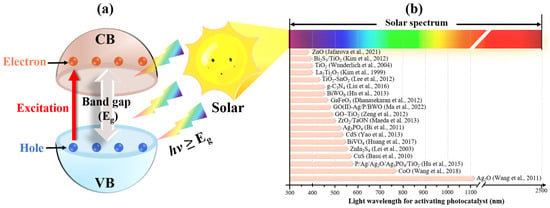
2. Feasibility of Solar Energy for Photocatalytic Applications
The formation of electrons and holes in photocatalysts requires photon excitation with sufficient energy. In order to excite electrons from the valence band (VB) of the photocatalyst to the conduction band (CB), the photon energy carried by the incident light needs to overcome the band gap between the VB and CB (as shown in Figure 1a). After decades of development, a large family of photocatalysts has been formed. According to the element composition, synthesis conditions and defect location, different photocatalysts have different band gaps. Figure 1b shows the light wavelength required for activating some typical photocatalysts (data from [8][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35][36][37]). It can be seen that although the sun emits light over a wide range of wavelengths, traditional photocatalysts such as TiO2 can only be excited by UV light with wavelengths less than 380 nm [28]. Since UV energy only accounts for a small proportion of solar energy, this results in low UV radiation intensity and low photocatalytic efficiency. In recent years, newly developed photocatalysts such as P/Ag/Ag2O/Ag3PO4/TiO2 composites [31] have expanded the utilizable solar radiation band, enabling it to be excited by both visible light and UV, significantly improving the photocatalytic efficiency compared to TiO2 alone. Since solar energy is an infinite and widely distributed light source possessing a broad spectrum wavelength ranging from 300 to 2500 nm [7], it could excite the photocatalyst with any band gap, thus implementing the photocatalytic reaction process.

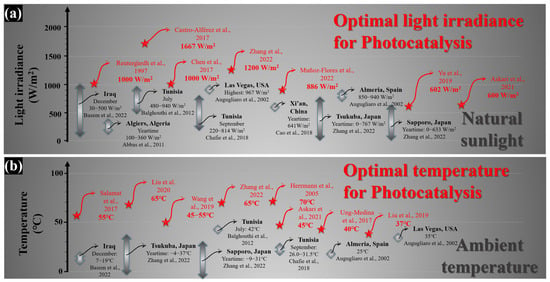
In addition, from a practical point of view, to make photocatalytic technology applicable, efficient photocatalytic processes need to be pursued. Therefore, suitable photocatalytic reaction conditions need to be provided and maintained. Since solar light energy mainly provides light irradiance and the reaction temperature, whether natural sunlight is qualified to meet the appropriate reaction conditions for efficient photocatalysis needs to be investigated. Figure 2 illustrates the optimal light irradiance required for various photocatalytic reactions. It can be observed that for a wide range of photocatalytic reactions, including the removal of organic dyes (rhodamine b, tartrazine, reactive black 5, etc.) [7][38][39], the degradation of harmful antibiotics (metronidazole, tetracycline, cephalexin, etc.) [40][41], the inactivation of pathogenic bacteria (Escherichia coli, etc.) [42] and the oxidation of organic matters (aerobic oxidation, etc.) [43], the light irradiance in the range of 600–1600 W/m2 is conducive to the implementation of efficient photocatalytic reactions (Figure 2a). However, under real environmental conditions, the solar light irradiance (data from [7][44][45][46][47][48][49]) is much lower than the appropriate light irradiance required for efficient photocatalysis (Figure 2a).

Figure 2. Comparison of optimum photocatalytic reaction conditions with natural conditions. (a) Light irradiance [7][38][39][40][41][42][43][44][45][46][47][48][49]; (b) temperature [7][10][40][45][46][47][49][50][51][52][53][54].
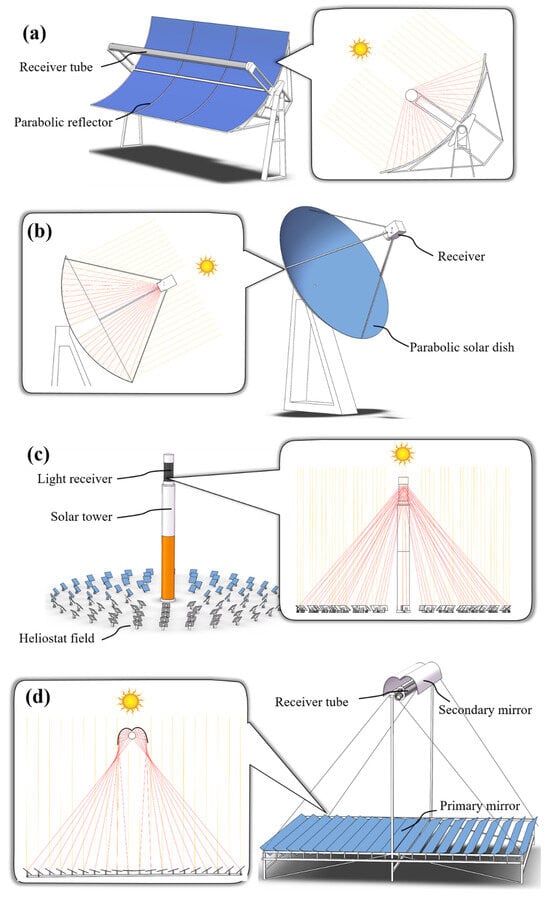
3. Brief Overview of Concentrated Solar Power Technologies
3.1. Parabolic Trough Reflectors
The PTR might be the most common and widely deployed CSP technology. As shown in Figure 3a, the most important technical feature of the PTR is that it is equipped with a parabolic-shaped concentrator mirror and a receiver tube located at the focal line of the parabola [45]. Owing to the optical properties of the paraboloid, it can efficiently focus incident parallel sunlight onto its focal line. This capability allows the receiver to harness a substantial amount of light energy, subsequently converting it into a concentrated heat flux. In order for the parabolic mirror to accurately converge parallel solar rays, the aperture of the parabola must always face the incidence direction of sunlight accurately. Therefore, parabolic mirrors are usually equipped with a controlling system to track solar motion. Due to its simpler structure compared with other CSP technologies, PTR has been successfully applied in various application scenarios. The most interesting application of PTR might be in the field of solar thermal power generation [55]. By concentrating a large amount of light and heat energy, the thermal medium (such as thermal oil and molten salts) in the receiver tube is heated, thus heating the water into high-temperature steam, which drives the turbine to generate electricity. Additionally, the solar cooking system also consists of an aluminum reflector with a vacuum tube, which provides a suitable cooking temperature of over 200 °C under actual solar conditions [56].

Figure 3. Schematic diagram of major concentrated solar power (CSP) technologies. (a) Parabolic trough reflector (PTR); (b) parabolic dish reflector (PDR); (c) solar power tower (SPT) and (d) linear Fresnel reflector (LFR).
3.2. Parabolic Dish Reflectors
The main components of a PDR system (shown in Figure 3b) include a parabolic dish concentrator mirror and a thermal receiver mounted at the focus point of the parabolic dish [57]. Similar to PTR technology, PDR also requires the incident direction of the parallel sunlight to be perpendicular to the aperture of the parabolic dish, thus enabling effective solar energy harvesting. Therefore, a two-axis sun tracking system is also indispensable for PDR. Generally, PDR systems are characterized by a high efficiency, autonomous operation and excellent modularity [57]. Many PDR-based power plants have been manufactured worldwide, which exhibit high solar-to-electric conversion efficiency and have the potential to become one of the most effective renewable energy utilization approaches [44][49][57].
3.3. Solar Power Towers
In recent decades, as a representative of high-temperature concentration engineering, SPT has been a popular topic in CSP engineering applications [58]. As shown in Figure 3c, in the SPT system, a large number of heliostats are arranged in an oval shape around the heat collecting tower. The heliostat could automatically track the movement of the sun and focus sunlight to the receiver located above the tower, thereby converting light energy into heat flux. The solar concentration ratio of a SPT can reach the equivalent of hundreds to thousands of suns, thereby achieving ultra-high operating temperatures. In recent years, to achieve the goal of saving energy and reducing emissions, SPT-based photo-thermal power plants employing the Rankine or Brayton cycle have been intensively studied and deployed [59].
3.4. Linear Fresnel Reflectors
In the past few decades, LFRs have also been studied and developed, and they have been widely used in photo-thermal power generation projects [60][61]. As shown in Figure 3d, the main structure of an LFR includes segmented flat mirrors which are mounted in parallel (as a primary mirror) and a secondary mirror located above the primary mirror array. The light receiver is mounted at the focus position of the secondary mirror. Each row of primary mirrors has a rotation axis, and all the primary mirrors track the position of the sun in the sky under the control of the driving mechanism, focusing the sunlight on the secondary mirror [7]. The shape of the secondary mirror is a compound parabolic collector (CPC) that can capture and reflect incoming sunlight in all directions to its focal point [62]. CPC, as a supporting CSP technology for LFR, plays a crucial role in improving the efficiency and heat flow uniformity of LFR systems. CPC is usually formed by a variety of shape combinations (such as involute, parabola, etc.), thus endowing it with the unique capability to collect beam radiation and diffuse radiation within a specific acceptable half-angle and without the utilization of a complex solar tracking system [63].
4. CSP Technologies Applied in Photocatalysis
4.1. Solar Concentrated Type
4.1.1. Parabolic Trough Reflector-Based Photoreactors
At present, solar-concentrated types of photoreactors mainly apply three technologies, including PTR, PDR and LFR. As a compact and efficient configuration for harvesting solar energy, it is not surprising that PTRs have been widely adopted as the basis for the construction of photocatalytic reactors [64][65][66][67][68][69][70][71][72]. As early as 1991, Anderson et al. reported a PTR-based photocatalytic reactor for groundwater remediation, which was widely considered to be the first on-site application project [72]. This project was built at the Lawrence Livermore Laboratory in the United States. The system structure consisted of rows of parabola troughs arranged in a field to form a trough array, which could reflect solar rays onto the reaction tube filled with photocatalyst particles. Subsequently, from 1993, a series of PTR-based photoreactors employing TiO2 as a photocatalyst for the degradation of various organic pollutants was tested and evaluated at the Plataforma Solar de Almeria (PSA), Spain. For example, Minero et al. established a large solar plant for the photocatalytic degradation of pentachlorophenol, which achieved a water treatment capacity of cubic meters per hour [68]. The system consisted of a total of twelve heliostat modules that could track solar motion, and every heliostat module was equipped with four PTRs. The nominal aperture area of each heliostat module was 32 m2, thus providing sufficient light energy for the reaction tube. They also carried out the photocatalytic degradation of atrazine by employing one of the heliostat modules in the above solar plant, which exhibited an atrazine removal ratio of 98% in 2 h. Other photocatalytic experiments using PTR-based photoreactors include the inactivation of Escherichia coli [65], the elimination of methylene blue and rhodamine b [70] and the removal of oxalic acid [71].
4.1.2. Parabolic Dish Reflector-Based Photoreactors
Due to the high efficiency and compactness of PDR technology in concentrating solar energy, unique PDR-based photoreactors have also been explored. Oyama et al. presented a batch-mode photoreactor system aiming to achieve the photocatalytic degradation of commercial detergent under real sunlight [73]. The system consisted of a round concave mirror (parabolic dish reflector) with an aperture diameter of 1.0 m and a flask-type reaction vessel. The parabolic dish reflector employed could achieve a geometric concentration ratio of 70 suns. The reaction vessel was mounted at the focal point of the parabolic dish for adsorbing the incident solar energy. TiO2, as the photocatalyst, was used in suspension and flowed inside the system circulation loop. The photocatalytic degradation of the refractory detergent driven by concentrated sunlight exhibited a much higher treatment efficiency than conventional biodegradation.
4.1.3. Fresnel Condenser-Based Photoreactors
Linear Fresnel reflectors, due to their excellent solar energy harvesting capabilities and widely acknowledged low costs, have also shown strengths in the structural exploration of photocatalytic systems. Zhang et al. developed an LFR-based photoreactor for the photocatalytic treatment of various organic contaminants including organic dye, antibiotics and pathogenic microorganisms [7]. In this system, six flat primary mirrors were mounted on the pedestal of the reactor and driven by stepper motors. The system was located in the north–south direction, with the primary continuously tracking and reflecting solar rays to the reaction tubes. The quartz glass reaction tubes were located above the primary mirror array, which could be moved in a north–south direction to accommodate the solar altitude angle in different seasons. Different from previous studies employing TiO2 particles, this LFR-based photoreactor utilized TiO2-coated silicone beads as the photocatalyst, which filled the inside of the reaction tubes. Rhodamine b, amoxicillin and Escherichia coli were successfully eliminated under actual weather conditions including a sunny day, cloudy day and rainy day, which demonstrated the feasibility of the LFR configuration for providing solar energy to photocatalysis.
4.1.4. Problems in Solar-Concentrated Photoreactors
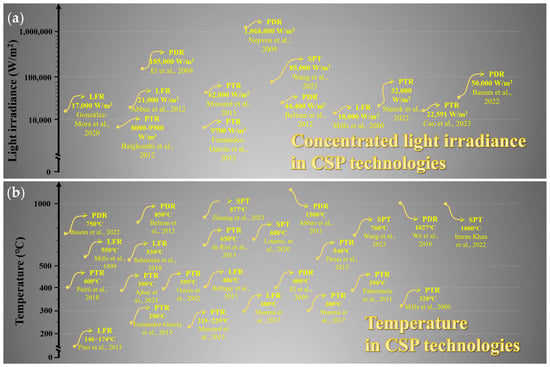
Although solar energy-concentrated photocatalytic reactors have demonstrated the capability of improving photocatalytic efficiency, their inherent disadvantages appeared with further research. First, solar-concentrated photoreactors may provide excessively high light irradiance and reaction temperatures for photocatalytic reactions [17][74]. Figure 4a shows the ability of some typical PTRs [45][75][76][77][78], PDRs [49][79][80][81], SPTs [59] and LFRs [60][62][82] to concentrate solar energy. Usually, these solar concentrators are used to concentrate solar energy efficiently to produce high temperatures which can be employed in thermal engineering processes such as in photo-thermal power plants. A solar concentration ratio equivalent to hundreds or thousands of suns was achieved, resulting in ultra-high light irradiance (Figure 4a).

Figure 4. Solar thermal energy provided by typical CSP technologies. (a) Concentrated light irradiance [45][49][59][60][62][75][76][77][78][79][80][81][82]; (b) temperature [18][19][44][49][57][58][59][61][62][75][77][79][81][83][84][85][86][87][88][89][90][91][92].
Figure 4b illustrates the temperature in some typical CSP projects, including PTR [18][19][62][75][77][83][84][85][86][87], PDR [44][49][57][79][81], SPT [58][59][88][89] and LFR [61][84][90][91][92]. For example, de Risi et al. optimized a PTR-based solar collector, which achieved a nanofluid outlet temperature of 650 °C [87]. Khan et al. introduced a next-generation SPT system with a temperature of more than 1000 °C [58]. Additionally, a dish Stirling system was developed by Abbas et al., who demonstrated its ability to achieve a temperature in excess of 1500 °C [44].
Secondly, although PTR, PDR, SPT and LFR can achieve effective solar energy convergence under sunny conditions with sufficient direct light, their photothermal harvesting capability will plummet on cloudy days, thus greatly limiting the efficiency of the photocatalytic reaction [1][7]. Thirdly, for these solar concentrator configurations, complex mechanisms for driving these structures are unavoidable due to the need to actively track the incidence angle of the solar rays [17]. This would increase the overall design difficulty, construction investment, operation and maintenance costs of photocatalytic reactors. Due to the above issues, traditional CSP-based photoreactors, mainly PTRs, are widely considered to be an outdated technology. In order to find photoreactors that can achieve appropriate enhancement of natural solar energy for achieving promoted photocatalytic efficiency, non/low-concentrated photoreactors began to be developed.
4.2. Non/Low-Concentrated Photoreactors
4.2.1. V-Groove-Based Photoreactors
The V-groove, as a variant of the PTR, simplifies the profile of the parabola into two flat mirrors, thereby dramatically reducing the solar concentrating ratio. V-groove-based photoreactors were mainly studied in 2004 by McLoughlin et al. and Bandala et al. to explore the advantages and disadvantages of PTR, V-groove and CPC technologies in photocatalytic applications [65][71]. TiO2 was adopted as the photocatalyst for the degradation of various organic targets including oxalic acid and Escherichia coli under real sunlight. Due to the low concentration of sunlight, the reaction temperature of the V-groove-based photoreactor never exceeded 38 °C. In comparison experiments, the CPC-based photoreactor clearly demonstrated a higher photocatalytic efficiency compared to the V-groove-based photoreactor. This may be due to the fact that the V-groove has no optical focus; therefore, the flat mirror cannot effectively provide the reflected light to the reaction tube, thereby resulting in its low efficiency. As a transitional photocatalytic reactor technology, the V-groove-based photoreactor was quickly eliminated by CPC-based photoreactors.
4.2.2. Compound Parabolic Collector-Based Photoreactors
As mentioned above, in traditional applications, CPC was used as a secondary mirror for LFR, which has the unique advantage of being able to focus the light incoming from any direction to its focus point [17]. Applying a similar principle, under sunny conditions, a fixed CPC could focus direct solar light with different incidence angles at its focal point or reflect scattered light at its focal point on cloudy days. Due to the excellent adaptability of CPC to actual weather conditions, currently, CPC-based photoreactors have gradually become the mainstream configuration of photocatalytic reactors, and a large number of CPC photoreactor experiments have been conducted [38][46][48][63][65][69][71][93][94][95][96][97][98][99][100][101][102][103][104][105][106][107][108][109].
Afterwards, another CPC-based treatment plant was established by Vidal et al. for detoxification and disinfection of contaminated water [93]. Various typical organic pollutants such as pesticides (EPTC, butiphos and γ-lindane) and microorganisms (Escherichia coli, Enterococcus faecalis) were selected as the treatment targets. All the organic contaminants were nearly 100% removed after 30 min of sunlight exposure, further demonstrating the applicability of CPC photoreactors to different treatment targets. Furthermore, a variant of CPC photoreactors has also been explored to promote its photocatalytic reaction performance.
5. Relevant Problems in CSP-Based Photoreactors
5.1. Instability of Real Weather
Although solar energy is an ideal energy source that is widely distributed and could be utilized at a low cost, the actual weather inevitably causes instability in the natural solar energy reaching the ground [7]. For the photocatalytic process, appropriate light irradiance is required to maximize the excitation of the photocatalyst and favorable heat energy is required to promote the reaction rate [7]. Therefore, natural variations in solar energy could inevitably affect the photocatalytic reaction rate. It also needs to be pointed out that modern industrial processes pursue the high robustness of system operation, and uncontrolled decline in the production rate is usually unacceptable. Therefore, if solar-energy-driven photocatalysis technology is to be truly extended to large-scale industrial processes (such as wastewater treatment, hydrogen production, biomass conversion, etc.), searching for solutions that could mitigate or even avoid the effects of natural solar variability on photocatalytic rates needs to be prioritized.
At present, some efforts have been put into solving this problem by researchers. In the development of a photocatalytic reactor based on a linear Fresnel concentrator constructed by Zhang et al., a novel solar energy regulation strategy was employed [7]. Traditional linear Fresnel concentrators utilize multiple primary mirrors to track the sun’s movement and reflect the sunlight to the focus to obtain ultra-high energy. Meanwhile, in their reactors, the innovation is that each primary mirror was controlled to rotate through its own independent control system. Under different weather conditions and at different times during the day, there were different numbers of primary mirrors used to focus sunlight. On sunny days when natural sunlight is strong, only two or three primary mirrors focus sunlight on the photocatalytic reaction tube, thus providing superior light irradiance and reaction temperatures for maximizing photocatalytic efficiency.
5.2. Nighttime Operation
Photocatalytic reactions require continuous input of light and heat energy to proceed smoothly. Considering the requirement of high robustness for actual industrial process, it is also a challenge to maintain efficient photocatalytic processes at night when there is no natural sunlight. The adoption of artificial light sources for nighttime photocatalytic lighting is straightforward in theory, but in the actual engineering project design, it is necessary to integrate the natural sunlight collector with the artificial light source.
5.3. Configuration of Photocatalyst Immobilization Substrate
Most CSP-based photoreactors utilize suspended photocatalysts, which may lead to complex post-separation and increased operating costs. In order to facilitate the large-scale application of photocatalytic technology, the immobilized photocatalyst needs to be equipped in the photoreactors.
In the previous studies, Villén et al. tested the efficiency of two CPC prototypes with different substrate configurations of photocatalysts, namely coaxial- and a fin-type photocatalysts [97]. The results demonstrated that the fin-type photochemical reactor always showed higher inactivation of waterborne bacteria than the coaxial-type reactor. This phenomenon might be due to the lower moving rate of water flowing through the fin-type system, which may lead to a higher number of bacteria attached to the immobilized photocatalyst.
It is worth noting that the above-mentioned development and optimization of the supporting configuration of the immobilized photocatalysts are all oriented towards specific application scenarios (e.g., sterilization and microalgae processing). The conclusions obtained may not be applicable to a wide range of application scenarios. For other application scenarios (such as gas-phase treatment, water treatment, water splitting, etc.) and different working conditions (such as reactant concentrations, flow rates, etc.), appropriate catalyst substrate configurations are needed. Generally, in a CSP-based photoreactor, the immobilization of photocatalyst may need to meet the following requirements: (1) achieve effective incident light energy utilization; (2) avoid obstructing the flow of the reactant and (3) load a large amount of photocatalyst. Based on the above requirements, an immobilization substrate and configuration that are suitable for CSP-based photocatalytic reactors need to be designed correspondingly.
5.4. Economic Analysis
An economic evaluation is one of the criteria used to judge whether a new technology can be applied to practical applications. Due to the absence of practical application examples of large-scale photocatalytic facilities, an economic analysis of photocatalytic reactors is required. In a study by Vidal et al., a large-scale CPC photocatalytic wastewater treatment plant with an occupied area of 500 m2 was assumed [93]. The operation cost of this plant was estimated to be 0.7 USD for 1 m3 water treatment (based on 1997 construction cost indices), which exhibited excellent cost-effectiveness compared with conventional technologies (around 1.0 dollars/m3). Then, Vela et al. carried out an economic comparison of CPC photoreactors using different photocatalysts (ZnO and TiO2) [107]. Assuming 365 sunny days in a year and 8 h of system operation per day, the cost per cubic meter of water treated by the TiO2-adopted CPC photoreactor was 1.45-fold higher than that of the ZnO system. The difference in process cost caused by the different catalysts was mainly due to the different times the catalysts required for pollutants degradation.
6. Conclusions
Efficient photocatalytic reactions require appropriate light irradiance and temperatures, and the low energy density of natural solar energy highlights the necessity of combining CSP technology with photocatalysis. Although existing CSP-based photoreactor technology has significantly improved the practical availability of photocatalysis, the challenges in unstable real weather, nighttime operation, post-separation and economic concern remain to be solved. Based on the latest reports, the adoption of a solar energy control strategy, employment of UV-visible responsive photocatalyst and immobilization substrate, innovations in reactor structure, economic evaluation of systems and establishment of reactor industry standards may be favorable for technological breakthroughs and engineering applications of future CSP-based photoreactors.
References
- Zhang, C.; Ming, J.; Sun, X.; Zhu, Y.; An, G.; Chen, G.; Yang, Y. Development of a Green and Efficient Photocatalytic Mesh Microalgae Biorefinery (PMMB) System for Sustainable Biomass Conversion under Real Solar Light. Chem. Eng. J. 2023, 466, 143260.
- Akpan, J.O.O. Sustainable Energy Development: History and Recent Advances. Energies 2023, 16, 7049.
- Ming, J.; Sun, X.; Ma, Q.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Advanced Photocatalytic Sterilization for Recalcitrant Enterococcus Sp. Contaminated Water by Newly Developed Z-Scheme Bi2WO6 Based Composites under Solar Light. Chemosphere 2023, 310, 136912.
- Ma, Q.; Hu, X.; Liu, N.; Sharma, A.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Polyethylene Glycol (PEG)-Modified Ag/Ag2O/Ag3PO4/Bi2WO6 Photocatalyst Film with Enhanced Efficiency and Stability under Solar Light. J. Colloid Interface Sci. 2020, 569, 101–113.
- Zhu, Q.; Liu, N.; Ma, Q.; Sharma, A.; Nagai, D.; Sun, X.; Zhang, C.; Yang, Y. Sol-Gel/Hydrothermal Two-Step Synthesis Strategy for Promoting Ag Species–Modified TiO2-Based Composite Activity toward H2 Evolution under Solar Light. Mater. Today Energy 2021, 20, 100648.
- Hu, X.; Ma, Q.; Wang, X.; Yang, Y.; Liu, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Layered Ag/Ag2O/BiPO4/Bi2WO6 Heterostructures by Two-Step Method for Enhanced Photocatalysis. J. Catal. 2020, 387, 28–38.
- Zhang, C.; Liu, N.; Ming, J.; Sharma, A.; Ma, Q.; Liu, Z.; Chen, G.; Yang, Y. Development of a Novel Solar Energy Controllable Linear Fresnel Photoreactor (LFP) for High-Efficiency Photocatalytic Wastewater Treatment under Actual Weather. Water Res. 2022, 208, 117880.
- Bi, Y.; Ouyang, S.; Umezawa, N.; Cao, J.; Ye, J. Facet Effect of Single-Crystalline Ag3PO4 Sub-Microcrystals on Photocatalytic Properties. J. Am. Chem. Soc. 2011, 4, 6490–6492.
- Portela, R.; Suárez, S.; Tessinari, R.F.; Hernández-Alonso, M.D.; Canela, M.C.; Sánchez, B. Solar/Lamp-Irradiated Tubular Photoreactor for Air Treatment with Transparent Supported Photocatalysts. Appl. Catal. B Environ. 2011, 105, 95–102.
- Liu, N.; Zhu, Q.; Zhang, N.; Zhang, C.; Kawazoe, N.; Chen, G.; Negishi, N.; Yang, Y. Superior Disinfection Effect of Escherichia Coli by Hydrothermal Synthesized TiO2-Based Composite Photocatalyst under LED Irradiation: Influence of Environmental Factors and Disinfection Mechanism. Environ. Pollut. 2019, 247, 847–856.
- Zhang, G.; Lan, Z.A.; Lin, L.; Lin, S.; Wang, X. Overall Water Splitting by Pt/g-C3N4 Photocatalysts without Using Sacrificial Agents. Chem. Sci. 2016, 7, 3062–3066.
- Liu, J.; Liu, Y.; Liu, N.; Han, Y.; Zhang, X.; Huang, H.; Lifshitz, Y.; Lee, S.T.; Zhong, J.; Kang, Z. Metal-Free Efficient Photocatalyst for Stable Visible Water Splitting via a Two-Electron Pathway. Science 2015, 347, 970–974.
- Reli, M.; Edelmannová, M.; Šihor, M.; Praus, P.; Svoboda, L.; Mamulová, K.K.; Otoupalíková, H.; Čapek, L.; Hospodková, A.; Obalová, L.; et al. Photocatalytic H2 Generation from Aqueous Ammonia Solution Using ZnO Photocatalysts Prepared by Different Methods. Int. J. Hydrogen Energy 2015, 40, 8530–8538.
- Kominami, H.; Nishimune, H.; Ohta, Y.; Arakawa, Y.; Inaba, T. Photocatalytic Hydrogen Formation from Ammonia and Methyl Amine in an Aqueous Suspension of Metal-Loaded Titanium(IV) Oxide Particles. Appl. Catal. B Environ. 2012, 111–112, 297–302.
- Vidyasagar, D.; Ghugal, S.G.; Kulkarni, A.; Mishra, P.; Shende, A.G.; Jagannath; Umare, S.S.; Sasikala, R. Silver/Silver(II) Oxide (Ag/AgO) Loaded Graphitic Carbon Nitride Microspheres: An Effective Visible Light Active Photocatalyst for Degradation of Acidic Dyes and Bacterial Inactivation. Appl. Catal. B Environ. 2018, 221, 339–348.
- Deng, J.; Liang, J.; Li, M.; Tong, M. Enhanced Visible-Light-Driven Photocatalytic Bacteria Disinfection by g-C3N4-AgBr. Colloids Surf. B Biointerfaces 2017, 152, 49–57.
- Braham, R.J.; Harris, A.T. Review of Major Design and Scale-up Considerations for Solar Photocatalytic Reactors. Ind. Eng. Chem. Res. 2009, 48, 8890–8905.
- Ajbar, W.; Hernández, J.A.; Parrales, A.; Torres, L. Thermal Efficiency Improvement of Parabolic Trough Solar Collector Using Different Kinds of Hybrid Nanofluids. Case Stud. Therm. Eng. 2023, 42, 102759.
- Desai, N.B.; Bandyopadhyay, S. Optimization of Concentrating Solar Thermal Power Plant Based on Parabolic Trough Collector. J. Clean. Prod. 2015, 89, 262–271.
- Ma, Q.; Ming, J.; Sun, X.; Liu, N.; Chen, G.; Yang, Y. Visible Light Active Graphene Oxide Modified Ag/Ag2O/BiPO4/Bi2WO6 for Photocatalytic Removal of Organic Pollutants and Bacteria in Wastewater. Chemosphere 2022, 306, 135512.
- Jafarova, V.N.; Orudzhev, G.S. Structural and Electronic Properties of ZnO: A First-Principles Density-Functional Theory Study within LDA(GGA) and LDA(GGA)+U Methods. Solid State Commun. 2021, 325, 114166.
- Huang, C.K.; Wu, T.; Huang, C.W.; Lai, C.Y.; Wu, M.Y.; Lin, Y.W. Enhanced Photocatalytic Performance of BiVO4 in Aqueous AgNO3 Solution under Visible Light Irradiation. Appl. Surf. Sci. 2017, 399, 10–19.
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Electrospun TiO2/SnO2 Nanofibers with Innovative Structure and Chemical Properties for Highly Efficient Photocatalytic H2 Generation. Int. J. Hydrogen Energy 2012, 37, 10575–10584.
- Yao, W.; Song, X.; Huang, C.; Xu, Q.; Wu, Q. Enhancing Solar Hydrogen Production via Modified Photochemical Treatment of Pt/CdS Photocatalyst. Catal. Today 2013, 199, 42–47.
- Zeng, P.; Zhang, Q.; Zhang, X.; Peng, T. Graphite Oxide-TiO2 Nanocomposite and Its Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. J. Alloys Compd. 2012, 516, 85–90.
- Kim, J.; Kang, M. High Photocatalytic Hydrogen Production over the Band Gap-Tuned Urchin-like Bi2S3-Loaded TiO2 Composites System. Int. J. Hydrogen Energy 2012, 37, 8249–8256.
- Liu, J.; Cheng, B.; Yu, J. A New Understanding of the Photocatalytic Mechanism of the Direct Z-Scheme g-C3N4/TiO2 Heterostructure. Phys. Chem. Chem. Phys. 2016, 18, 31175–31183.
- Wunderlich, W.; Oekermann, T.; Miao, L.; Hue, N.T.; Tanemura, S.; Tanemura, M. Electronic Properties of Nano-Porous TiO2- and ZnO-Thin Films-Comparison of Simulations and Experiments. J. Ceram. Process. Res. 2004, 5, 343–354.
- Lei, Z.; You, W.; Liu, M.; Zhou, G.; Takata, T.; Hara, M.; Domen, K.; Li, C. Photocatalytic Water Reduction under Visible Light on a Novel ZnIn2S4 Catalyst Synthesized by Hydrothermal Method. Chem. Commun. 2003, 3, 2142–2143.
- Wang, X.; Li, S.; Yu, H.; Yu, J.; Liu, S. Ag2O as a New Visible-Light Photocatalyst: Self-Stability and High Photocatalytic Activity. Chem. A Eur. J. 2011, 17, 7777–7780.
- Hu, X.; Zhu, Q.; Wang, X.; Kawazoe, N.; Yang, Y. Nonmetal-Metal-Semiconductor-Promoted P/Ag/Ag2O/Ag3PO4/TiO2 Photocatalyst with Superior Photocatalytic Activity and Stability. J. Mater. Chem. A 2015, 3, 17858–17865.
- Maeda, K.; Lu, D.; Domen, K. Direct Water Splitting into Hydrogen and Oxygen under Visible Light by Using Modified Taon Photocatalysts with D0 Electronic Configuration. Chem. A Eur. J. 2013, 19, 4986–4991.
- Kim, H.G.; Hwang, D.W.; Kim, J.; Kim, Y.G.; Lee, J.S. Highly Donor-Doped (110) Layered Perovskite Materials as Novel Photocatalysts for Overall Water Splitting. Chem. Commun. 1999, 2, 1077–1078.
- Dhanasekaran, P.; Gupta, N.M. Factors Affecting the Production of H2 by Water Splitting over a Novel Visible-Light-Driven Photocatalyst GaFeO3. Int. J. Hydrogen Energy 2012, 37, 4897–4907.
- Wang, Y.; Ge, H.X.; Chen, Y.P.; Meng, X.Y.; Ghanbaja, J.; Horwat, D.; Pierson, J.F. Wurtzite CoO: A Direct Band Gap Oxide Suitable for a Photovoltaic Absorber. Chem. Commun. 2018, 54, 13949–13952.
- Hu, S.P.; Xu, C.Y.; Zhen, L. Solvothermal Synthesis of Bi2WO6 Hollow Structures with Excellent Visible-Light Photocatalytic Properties. Mater. Lett. 2013, 95, 117–120.
- Basu, M.; Sinha, A.K.; Pradhan, M.; Sarkar, S.; Negishi, Y.; Govind; Pal, T. Evolution of Hierarchical Hexagonal Stacked Plates of CuS from Liquid—Liquid Interface and Its Photocatalytic Application for Oxidative Degradation of Different Dyes under Indoor Lighting. Environ. Sci. Technol. 2010, 44, 6313–6318.
- Muñoz-Flores, P.; Poon, P.S.; Ania, C.O.; Matos, J. Performance of a C-Containing Cu-Based Photocatalyst for the Degradation of Tartrazine: Comparison of Performance in a Slurry and CPC Photoreactor under Artificial and Natural Solar Light. J. Colloid Interface Sci. 2022, 623, 646–659.
- Reutergårdh, L.B.; Iangphasuk, M. Photocatalytic Decolourization of Reactive Azo Dye: A Comparison between TiO2 and CdS Photocatalysis. Chemosphere 1997, 35, 585–596.
- Askari, N.; Beheshti, M.; Mowla, D.; Farhadian, M. Facile Construction of Novel Z-Scheme MnWO4/Bi2S3 Heterojunction with Enhanced Photocatalytic Degradation of Antibiotics. Mater. Sci. Semicond. Process. 2021, 127, 105723.
- Yu, J.; Kiwi, J.; Zivkovic, I.; Rønnow, H.M.; Wang, T.; Rtimi, S. Quantification of the Local Magnetized Nanotube Domains Accelerating the Photocatalytic Removal of the Emerging Pollutant Tetracycline. Appl. Catal. B Environ. 2019, 248, 450–458.
- Castro-Alférez, M.; Polo-López, M.I.; Marugán, J.; Fernández-Ibáñez, P. Mechanistic Model of the Escherichia Coli Inactivation by Solar Disinfection Based on the Photo-Generation of Internal ROS and the Photo-Inactivation of Enzymes: CAT and SOD. Chem. Eng. J. 2017, 318, 214–223.
- Chen, Y.; Wang, Y.; Li, W.; Yang, Q.; Hou, Q.; Wei, L.; Liu, L.; Huang, F.; Ju, M. Enhancement of Photocatalytic Performance with the Use of Noble-Metal-Decorated TiO2 Nanocrystals as Highly Active Catalysts for Aerobic Oxidation under Visible-Light Irradiation. Appl. Catal. B Environ. 2017, 210, 352–367.
- Abbas, M.; Boumeddane, B.; Said, N.; Chikouche, A. Dish Stirling Technology: A 100 MW Solar Power Plant Using Hydrogen for Algeria. Int. J. Hydrogen Energy 2011, 36, 4305–4314.
- Balghouthi, M.; Chahbani, M.H.; Guizani, A. Investigation of a Solar Cooling Installation in Tunisia. Appl. Energy 2012, 98, 138–148.
- Augugliaro, V.; Baiocchi, C.; Prevot, A.B.; García-López, E.; Loddo, V.; Malato, S.; Marcí, G.; Palmisano, L.; Pazzi, M.; Pramauro, E. Azo-Dyes Photocatalytic Degradation in Aqueous Suspension of TiO2 under Solar Irradiation. Chemosphere 2002, 49, 1223–1230.
- Chafie, M.; Ben Aissa, M.F.; Guizani, A. Energetic End Exergetic Performance of a Parabolic Trough Collector Receiver: An Experimental Study. J. Clean. Prod. 2018, 171, 285–296.
- Cao, F.; Wei, Q.; Liu, H.; Lu, N.; Zhao, L.; Guo, L. Development of the Direct Solar Photocatalytic Water Splitting System for Hydrogen Production in Northwest China: Design and Evaluation of Photoreactor. Renew. Energy 2018, 121, 153–163.
- Basem, A.; Moawed, M.; Abbood, M.H.; El-Maghlany, W.M. The Energy and Exergy Analysis of a Combined Parabolic Solar Dish—Steam Power Plant. Renew. Energy Focus 2022, 41, 55–68.
- Salamat, S.; Younesi, H.; Bahramifar, N. Synthesis of Magnetic Core-Shell Fe3O4@TiO2 Nanoparticles from Electric Arc Furnace Dust for Photocatalytic Degradation of Steel Mill Wastewater. RSC Adv. 2017, 7, 19391–19405.
- Ung-Medina, F.; Caudillo-Flores, U.; Correa-González, J.C.; Maya-Yescas, R.; Chávez-Parga, M.D.C.; Cortés, J.A. Use of an Annular Non-Sleeve Photoreactor for Photocatalytic Dye Degradation: Study of Temperature and Light Intensity Effects. Environ. Prog. Sustain. Energy 2017, 36, 1083–1088.
- Wang, H.; Sun, Y.; Wu, Y.; Tu, W.; Wu, S.; Yuan, X.; Zeng, G.; Xu, Z.J.; Li, S.; Chew, J.W. Electrical Promotion of Spatially Photoinduced Charge Separation via Interfacial-Built-in Quasi-Alloying Effect in Hierarchical Zn2In2S5/Ti3C2(O, OH)x Hybrids toward Efficient Photocatalytic Hydrogen Evolution and Environmental Remediation. Appl. Catal. B Environ. 2019, 245, 290–301.
- Liu, B.; Wu, H.; Parkin, I.P. New Insights into the Fundamental Principle of Semiconductor Photocatalysis. ACS Omega 2020, 5, 14847–14856.
- Herrmann, J.M. Heterogeneous Photocatalysis: State of the Art and Present Applications In honor of Pr. R.L. Burwell Jr. (1912–2003), Former Head of Ipatieff Laboratories, Northwestern University, Evanston (Ill). Top. Catal. 2005, 34, 49–65.
- Al-Soud, M.S.; Hrayshat, E.S. A 50 MW Concentrating Solar Power Plant for Jordan. J. Clean. Prod. 2009, 17, 625–635.
- Balzar, A.; Stumpf, P.; Eckhoff, S.; Ackermann, H.; Grupp, M. A Solar Cooker Using Vacuum-Tube Collectors with Integrated Heat Pipes. Sol. Energy 1996, 58, 63–68.
- Wu, S.Y.; Xiao, L.; Cao, Y.; Li, Y.R. A Parabolic Dish/AMTEC Solar Thermal Power System and Its Performance Evaluation. Appl. Energy 2010, 87, 452–462.
- Imran Khan, M.; Asfand, F.; Al-Ghamdi, S.G. Progress in Technology Advancements for next Generation Concentrated Solar Power Using Solid Particle Receivers. Sustain. Energy Technol. Assess. 2022, 54, 102813.
- Wang, Q.; Yao, Y.; Shen, Z.; Hu, M.; Yang, H. Concentrated Solar Power Tower Systems Coupled Locally with Spectrally Selective Coatings for Enhancement of Solar-Thermal Conversion and Economic Performance. Green Energy Resour. 2023, 1, 100001.
- González-Mora, E.; Dolores Durán García, M. Methodology for an Opto-Geometric Optimization of a Linear Fresnel Reflector for Direct Steam Generation. Energies 2020, 13, 355.
- Sebastián, A.; Abbas, R.; Valdés, M.; Casanova, J. Innovative Thermal Storage Strategies for Fresnel-Based Concentrating Solar Plants with East-West Orientation. Appl. Energy 2018, 230, 983–995.
- Mills, D.R.; Morrison, G.L. Compact Linear Fresnel Reflector Solar Thermal Powerplants. Sol. Energy 2000, 68, 263–283.
- Ochoa-Gutiérrez, K.S.; Tabares-Aguilar, E.; Mueses, M.Á.; Machuca-Martínez, F.; Li Puma, G. A Novel Prototype Offset Multi Tubular Photoreactor (OMTP) for Solar Photocatalytic Degradation of Water Contaminants. Chem. Eng. J. 2018, 341, 628–638.
- Anderson, J.V.; Link, H.; Bohn, M.; Gupta, B. Development of Solar Detoxification Technology in the USA—An Introduction. Sol. Energy Mater. 1991, 24, 538–549.
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Pentachlorophenol. Chemosphere 1993, 26, 2103–2119.
- Malato, S.; Blanco, J.; Richter, C.; Curco, D.; Gimenez, J. Low-Concentrating CPC Collectors for Photocatalytic Water Detoxification: Comparison with a Medium Concentrating Solar Collector. Water Sci. Technol. 1997, 35, 157–164.
- Klare, M.; Scheen, J.; Vogelsang, K.; Jacobs, H.; Broekaert, J.A.C. Degradation of Short-Chain Alkyl- and Alkanolamines by TiO2- and Pt/TiO2-Assisted Photocatalysis. Chemosphere 2000, 41, 353–362.
- Sano, T.; Negishi, N.; Takeuchi, K.; Matsuzawa, S. Degradation of Toluene and Acetaldehyde with Pt-Loaded TiO2 Catalyst and Parabolic Trough Concentrator. Sol. Energy 2004, 77, 543–552.
- Bandala, E.R.; Arancibia-Bulnes, C.A.; Orozco, S.L.; Estrada, C.A. Solar Photoreactors Comparison Based on Oxalic Acid Photocatalytic Degradation. Sol. Energy 2004, 77, 503–512.
- McLoughlin, O.A.; Kehoe, S.C.; McGuigan, K.G.; Duffy, E.F.; Al Touati, F.; Gernjak, W.; Oller Alberola, I.; Malato Rodríguez, S.; Gill, L.W. Solar Disinfection of Contaminated Water: A Comparison of Three Small-Scale Reactors. Sol. Energy 2004, 77, 657–664.
- Barzegar, M.H.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z.; Roy, V.A.L.; Heidari, H. S-Scheme Heterojunction g-C3N4/TiO2 with Enhanced Photocatalytic Activity for Degradation of a Binary Mixture of Cationic Dyes Using Solar Parabolic Trough Reactor. Chem. Eng. Res. Des. 2021, 174, 307–318.
- Minero, C.; Pelizzetti, E.; Malato, S.; Blanco, J. Large Solar Plant Photocatalytic Water Decontamination: Degradation of Atrazine. Sol. Energy 1996, 56, 411–419.
- Oyama, T.; Aoshima, A.; Horikoshi, S.; Hidaka, H.; Zhao, J.; Serpone, N. Solar Photocatalysis, Photodegradation of a Commercial Detergent in Aqueous TiO2 Dispersions under Sunlight Irradiation. Sol. Energy 2004, 77, 525–532.
- Malato, S.; Blanco, J.; Vidal, A.; Richter, C. Photocatalysis with Solar Energy at a Pilot-Plant Scale: An Overview. Appl. Catal. B Environ. 2002, 37, 1–15.
- Fernández-García, A.; Rojas, E.; Pérez, M.; Silva, R.; Hernández-Escobedo, Q.; Manzano-Agugliaro, F. A Parabolic-Trough Collector for Cleaner Industrial Process Heat. J. Clean. Prod. 2015, 89, 272–285.
- Cao, F.; Pang, J.; Gu, X.; Wang, M.; Shangguan, Y. Performance Simulation of Solar Trough Concentrators: Optical and Thermal Comparisons. Energies 2023, 16, 1673.
- Mussard, M.; Nydal, O.J. Charging of a Heat Storage Coupled with a Low-Cost Small-Scale Solar Parabolic Trough for Cooking Purposes. Sol. Energy 2013, 95, 144–154.
- Stanek, B.; Węcel, D.; Bartela, Ł.; Rulik, S. Solar Tracker Error Impact on Linear Absorbers Efficiency in Parabolic Trough Collector—Optical and Thermodynamic Study. Renew. Energy 2022, 196, 598–609.
- Beltran, R.; Velazquez, N.; Espericueta, A.C.; Sauceda, D.; Perez, G. Mathematical Model for the Study and Design of a Solar Dish Collector with Cavity Receiver for Its Application in Stirling Engines. J. Mech. Sci. Technol. 2012, 26, 3311–3321.
- Nepveu, F.; Ferriere, A.; Bataille, F. Thermal Model of a Dish/Stirling Systems. Sol. Energy 2009, 83, 81–89.
- El Ouederni, A.R.; Salah, M.B.; Askri, F.; Nasrallah, M.B.; Aloui, F. Experimental Study of a Parabolic Solar Concentrator. J. Renew. Energ. 2009, 12, 395–404.
- Abbas, R.; Montes, M.J.; Piera, M.; Martínez-Val, J.M. Solar Radiation Concentration Features in Linear Fresnel Reflector Arrays. Energy Convers. Manag. 2012, 54, 133–144.
- Geyer, M.; Lüpfert, E.; Osuna, R.; Nava, P.; Langenkamp, J.; Mandelberg, E. EUROTROUGH-Parabolic Trough Collector Developed for Cost Efficient Solar Power Generation. In Proceedings of the 11th International Symposium on Concentrating Solar Power and Chemical Energy Technologies, Zurich, Switzerland, 4–6 September 2002; Volume 7.
- Sharma, A.K.; Sharma, C.; Mullick, S.C.; Kandpal, T.C. GHG Mitigation Potential of Solar Industrial Process Heating in Producing Cotton Based Textiles in India. J. Clean. Prod. 2017, 145, 74–84.
- Palenzuela, P.; Zaragoza, G.; Alarcón-Padilla, D.C.; Guillén, E.; Ibarra, M.; Blanco, J. Assessment of Different Configurations for Combined Parabolic-Trough (PT) Solar Power and Desalination Plants in Arid Regions. Energy 2011, 36, 4950–4958.
- Peiró, G.; Prieto, C.; Gasia, J.; Jové, A.; Miró, L.; Cabeza, L.F. Two-Tank Molten Salts Thermal Energy Storage System for Solar Power Plants at Pilot Plant Scale: Lessons Learnt and Recommendations for Its Design, Start-up and Operation. Renew. Energy 2018, 121, 236–248.
- de Risi, A.; Milanese, M.; Laforgia, D. Modelling and Optimization of Transparent Parabolic Trough Collector Based on Gas-Phase Nanofluids. Renew. Energy 2013, 58, 134–139.
- Linares, J.I.; Montes, M.J.; Cantizano, A.; Sánchez, C. A Novel Supercritical CO2 Recompression Brayton Power Cycle for Power Tower Concentrating Solar Plants. Appl. Energy 2020, 263, 114644.
- Zhuang, X.; Wang, H.; Lu, H.; Yang, Z.; Guo, H. Numerical Investigation of Heat Transfer and Flow Characteristics of Supercritical CO2 in Solar Tower Microchannel Receivers at High Temperature. Energies 2023, 16, 6445.
- Pino, F.J.; Caro, R.; Rosa, F.; Guerra, J. Experimental Validation of an Optical and Thermal Model of a Linear Fresnel Collector System. Appl. Therm. Eng. 2013, 50, 1463–1471.
- Beltagy, H.; Semmar, D.; Lehaut, C.; Said, N. Theoretical and Experimental Performance Analysis of a Fresnel Type Solar Concentrator. Renew. Energy 2017, 101, 782–793.
- Mills, D.R.; Morrison, G.L. Modelling Study for Compact Fresnel Reflector Power Plant. Le J. De Phys. IV 1999, 9, Pr3-159–Pr3-165.
- Vidal, A.; Díaz, A.I.; El Hraiki, A.; Romero, M.; Muguruza, I.; Senhaji, F.; González, J. Solar Photocatalysis for Detoxification and Disinfection of Contaminated Water: Pilot Plant Studies. Catal. Today 1999, 54, 283–290.
- Kositzi, M.; Poulios, I.; Malato, S.; Caceres, J.; Campos, A. Solar Photocatalytic Treatment of Synthetic Municipal Wastewater. Water Res. 2004, 38, 1147–1154.
- Fernández, P.; Blanco, J.; Sichel, C.; Malato, S. Water Disinfection by Solar Photocatalysis Using Compound Parabolic Collectors. Catal. Today 2005, 101, 345–352.
- Augugliaro, V.; García-López, E.; Loddo, V.; Malato-Rodríguez, S.; Maldonado, I.; Marcì, G.; Molinari, R.; Palmisano, L. Degradation of Lincomycin in Aqueous Medium: Coupling of Solar Photocatalysis and Membrane Separation. Sol. Energy 2005, 79, 402–408.
- Villén, L.; Manjón, F.; García-Fresnadillo, D.; Orellana, G. Solar Water Disinfection by Photocatalytic Singlet Oxygen Production in Heterogeneous Medium. Appl. Catal. B Environ. 2006, 69, 1–9.
- Sichel, C.; Tello, J.; de Cara, M.; Fernández-Ibáñez, P. Effect of UV Solar Intensity and Dose on the Photocatalytic Disinfection of Bacteria and Fungi. Catal. Today 2007, 129, 152–160.
- Alrousan, D.M.A.; Polo-López, M.I.; Dunlop, P.S.M.; Fernández-Ibáñez, P.; Byrne, J.A. Solar Photocatalytic Disinfection of Water with Immobilised Titanium Dioxide in Re-Circulating Flow CPC Reactors. Appl. Catal. B Environ. 2012, 128, 126–134.
- Quiñones, D.H.; Álvarez, P.M.; Rey, A.; Beltrán, F.J. Removal of Emerging Contaminants from Municipal WWTP Secondary Effluents by Solar Photocatalytic Ozonation. A Pilot-Scale Study. Sep. Purif. Technol. 2015, 149, 132–139.
- Colina-Márquez, J.; Machuca-Martínez, F.; Puma, G.L. Modeling the Photocatalytic Mineralization in Water of Commercial Formulation of Estrogens 17-β Estradiol (E2) and Nomegestrol Acetate in Contraceptive Pills in a Solar Powered Compound Parabolic Collector. Molecules 2015, 20, 13354–13373.
- Otálvaro-Marín, H.L.; Mueses, M.A.; Crittenden, J.C.; Machuca-Martinez, F. Solar Photoreactor Design by the Photon Path Length and Optimization of the Radiant Field in a TiO2-Based CPC Reactor. Chem. Eng. J. 2017, 315, 283–295.
- Aguas, Y.; Hincapie, M.; Fernández-Ibáñez, P.; Polo-López, M.I. Solar Photocatalytic Disinfection of Agricultural Pathogenic Fungi (Curvularia Sp.) in Real Urban Wastewater. Sci. Total Environ. 2017, 607–608, 1213–1224.
- Haranaka-Funai, D.; Didier, F.; Giménez, J.; Marco, P.; Esplugas, S.; Machulek-Junior, A. Photocatalytic Treatment of Valproic Acid Sodium Salt with TiO2 in Different Experimental Devices: An Economic and Energetic Comparison. Chem. Eng. J. 2017, 327, 656–665.
- Moreira, N.F.F.; Narciso-da-Rocha, C.; Polo-López, M.I.; Pastrana-Martínez, L.M.; Faria, J.L.; Manaia, C.M.; Fernández-Ibáñez, P.; Nunes, O.C.; Silva, A.M.T. Solar Treatment (H2O2, TiO2-P25 and GO-TiO2 Photocatalysis, Photo-Fenton) of Organic Micropollutants, Human Pathogen Indicators, Antibiotic Resistant Bacteria and Related Genes in Urban Wastewater. Water Res. 2018, 135, 195–206.
- López, N.; Marco, P.; Giménez, J.; Esplugas, S. Photocatalytic Diphenhydramine Degradation under Different Radiation Sources: Kinetic Studies and Energetic Comparison. Appl. Catal. B Environ. 2018, 220, 497–505.
- Vela, N.; Calín, M.; Yáñez-Gascón, M.J.; Garrido, I.; Pérez-Lucas, G.; Fenoll, J.; Navarro, S. Solar Reclamation of Wastewater Effluent Polluted with Bisphenols, Phthalates and Parabens by Photocatalytic Treatment with TiO2/Na2S2O8 at Pilot Plant Scale. Chemosphere 2018, 212, 95–104.
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar Photocatalytic Degradation of Pesticides over TiO2-RGO Nanocomposites at Pilot Plant Scale. Sci. Total Environ. 2020, 737, 140286.
- Zheng, Q.; Aiello, A.; Choi, Y.S.; Tarr, K.; Shen, H.; Durkin, D.P.; Shuai, D. 3D Printed Photoreactor with Immobilized Graphitic Carbon Nitride: A Sustainable Platform for Solar Water Purification. J. Hazard. Mater. 2020, 399, 123097.
More
Information
Subjects:
Engineering, Chemical
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.5K
Revisions:
2 times
(View History)
Update Date:
23 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




