
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Violeta Popovici | -- | 1555 | 2024-02-22 09:54:43 | | | |
| 2 | Jason Zhu | -1 word(s) | 1554 | 2024-02-23 03:07:21 | | |
Video Upload Options
The primary treatment for autoimmune Diabetes Mellitus (Type 1 Diabetes Mellitus-T1DM) is insulin therapy. Unfortunately, a multitude of clinical cases has demonstrated that the use of insulin as a sole therapeutic intervention fails to address all issues comprehensively. Therefore, non-insulin adjunct treatment has been investigated and shown successful results in clinical trials. Various hypoglycemia-inducing drugs such as Metformin, glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, amylin analogs, and Sodium-Glucose Cotransporters 2 (SGLT-2) inhibitors, developed good outcomes in patients with T1DM. SGLT-2 inhibitors have remarkably improved the treatment of patients with diabetes by preventing cardiovascular events, heart failure hospitalization, and progression of renal disease. However, their pharmacological potential has not been explored enough.
1. Introduction
| SGLT-2 Inhibitor |
Active Ingredient(s) |
Daily Dose (mg) |
Brand Name | Company |
|---|---|---|---|---|
| Selective SGLT-2 Inhibitors | ||||
| Canagliflozin [14][15][16] |
Canagliflozin | 100 | Invokana | Janssen-Cilag International NV Beerse, Belgium |
| Canagliflozin + Metformin | 50/500 50/1000 150/500 150/1000 |
Invokamet | ||
| Canagliflozin + Metformin extended-release | Invokamet XR | |||
| Dapagliflozin [17][18][19][20][21] |
Dapagliflozin | 5 10 |
Forxiga | AstraZeneca AB Södertälje Sweden |
| Dapagliflozin + Metformin extended-release |
5/1000 5/850 |
Xigduo XR | ||
| Dapagliflozin + Saxagliptin | 5/10 | Qtern | ||
| Empagliflozin [22][23][24][25] |
Empagliflozin | 10 25 |
Jardiance | Boehringer Ingelheim International GmbH Ingelheim am Rhein, Germany |
| Empagliflozin + Linagliptin | 10/5 25/5 |
Glyxambi | ||
| Empagliflozin + Metformin | 5/1000 5/850 12.5/1000 12.5/850 |
Synjardy | ||
| Empagliflozin + Metformin extended-release |
25/1000 | Synjardy XR | ||
| Ertugliflozin [26][27] |
Ertugliflozin | 5 15 |
Steglatro | Merck Sharp & Dohme Haarlem, Netherland |
| Ertugliflozin + Metformin | 2.5/850 7.5/850 |
Segluromet | ||
| Ertugliflozin + Sitagliptin | 5/100 15/100 |
Steglujan | ||
| Bexagliflozin [28][29][30][31][32][33] |
Bexagliflozin | 20 | Brenzavvy | TheracosBio, LLC Marlborough, MA, USA |
| Ipragliflozin [34][35][36][37][38] |
Ipragliflozin | 25 50 |
Suglat | Astellas Pharma LTD, Addlestone, UK |
| Luseogliflozin [39][40][41][42][43][44] |
Luseogliflozin | 2.5 5 |
Lusefi | Taisho Pharmaceutical Holdings Co., Ltd., Tokyo, Japan |
| Tofogliflozin [45][46][47] |
Tofogliflozin | 20 40 |
Apleway | Chugai Pharmaceutical Co., Ltd., Tokyo, Japan |
| Remogliflozin [3][4][5][48][49] |
Remogliflozin | 100 | Remogliflozin etabonate |
GlaxoSmithKline plc, Brentford, UK Glenmark Pharmceuticals Ltd., Mumbai, India |
| Henagliflozin [6][7][8][50][51] |
Henagliflozin | 5 10 |
SHR3824 | Jiangsu Hengrui Pharmaceuticals Co., Ltd., Lianyungang, China |
| Dual SGLT-2 + SGLT-1 Inhibitors | ||||
| Sotagliflozin [52][53][54][55][56][57][58][59][60] |
Sotagliflozin | 200 400 |
Zynquista Inpefa |
Lexicon Pharmaceuticals, Inc., The Woodlands, TX, USA |
| Licogliflozin [10][11][12][13][61] |
Licogliflozin | No data | LIK-066 | Novartis AG Basel, Switzerland |
2. Benefits of SGLT-2 Inhibitors
2.1. Weight Loss
2.2. Heart Protection
2.3. Kidney Protection
3. Adverse Effects of SGLT-2 Inhibitors
3.1. Acute Kidney Injury (AKI)
3.2. Polyuria
3.3. Euglycemic Diabetic Ketoacidosis (DKA)
3.4. Genito-Urinary Tract Infections
3.5. Bone Fractures and Amputation Risk
4. SGLT-2 Inhibitors in the Therapy of DM Complications and Comorbidities
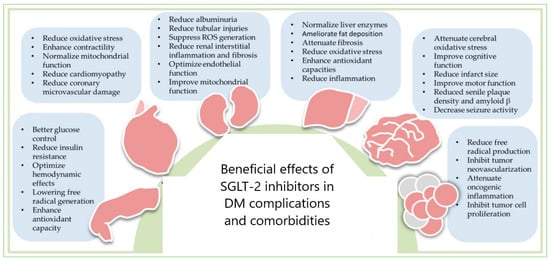
5. DM Patient Adherence to SGLT-2is Therapy
References
- Agrawal, P.; Pursnani, N.; Gautam, A.; Garg, R. Is REmogliflozin an Effective Drug in MANaging Type-2 Diabetes Mellitus: A Comparative Study—(REDMAN). Diabetes Epidemiol. Manag. 2022, 7, 100076.
- Dobbins, R.; Hussey, E.K.; O’Connor-Semmes, R.; Andrews, S.; Tao, W.; Wilkison, W.O.; Cheatham, B.; Sagar, K.; Hanmant, B. Assessment of Safety and Tolerability of Remogliflozin Etabonate (GSK189075) When Administered with Total Daily Dose of 2000 Mg of Metformin. BMC Pharmacol. Toxicol. 2021, 22, 34.
- Attimarad, M.; Venugopala, K.N.; Nair, A.B.; Sreeharsha, N.; Deb, P.K. Experimental Design Approach for Quantitative Expressions of Simultaneous Quantification of Two Binary Formulations Containing Remogliflozin and Gliptins by RP-HPLC. Separations 2022, 9, 23.
- Jain, R.; Bhavatharini, N.; Saravanan, T.; Seshiah, V.; Jain, N. Use of Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitor Remogliflozin and Possibility of Acute Kidney Injury in Type-2 Diabetes. Cureus 2022, 14, e32573.
- Que, L.; Huang, K.; Xiang, X.; Ding, Y.; Chu, N.; He, Q. No Apparent Pharmacokinetic Interactions Were Found between Henagliflozin: A Novel Sodium-glucose Co-transporter 2 Inhibitor and Glimepiride in Healthy Chinese Male Subjects. J. Clin. Pharm. Ther. 2022, 47, 1225–1231.
- He, X.; Liu, G.; Chen, X.; Wang, Y.; Liu, R.; Wang, C.; Huang, Y.; Shen, J.; Jia, Y. Pharmacokinetic and Pharmacodynamic Interactions Between Henagliflozin, a Novel Selective SGLT-2 Inhibitor, and Warfarin in Healthy Chinese Subjects. Clin. Ther. 2023, 45, 655–661.
- Lu, J.; Fu, L.; Li, Y.; Geng, J.; Qin, L.; Li, P.; Zheng, H.; Sun, Z.; Li, Y.; Zhang, L.; et al. Henagliflozin Monotherapy in Patients with Type 2 Diabetes Inadequately Controlled on Diet and Exercise: A Randomized, Double-blind, Placebo-controlled, Phase 3 Trial. Diabetes Obes. Metab. 2021, 23, 1111–1120.
- Zhang, Y.; Liu, Y.; Yu, C.; Wang, Y.; Zhan, Y.; Liu, H.; Zou, J.; Jia, J.; Chen, Q.; Zhong, D. Tolerability, Pharmacokinetic, and Pharmacodynamic Profiles of Henagliflozin, a Novel Selective Inhibitor of Sodium-Glucose Cotransporter 2, in Healthy Subjects Following Single- and Multiple-Dose Administration. Clin. Ther. 2021, 43, 396–409.
- Zaki, A.M.; Abo-Elnour, D.E.; Abdalla, Y.E.; Hassan, R.Y.; Salama, M.K.; Elboraay, T.; Abdelhaleem, I.A. Dose-Dependent Efficacy and Safety of Licogliflozin on Obese Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. Syndr. Clin. Res. Rev. 2022, 16, 102657.
- De Boer, R.A.; Núñez, J.; Kozlovski, P.; Wang, Y.; Proot, P.; Keefe, D. Effects of the Dual Sodium–Glucose Linked Transporter Inhibitor, Licogliflozin vs. Placebo or Empagliflozin in Patients with Type 2 Diabetes and Heart Failure. Br. J. Clin. Pharmacol. 2020, 86, 1346–1356.
- Tan, S.; Ignatenko, S.; Wagner, F.; Dokras, A.; Seufert, J.; Zwanziger, D.; Dunschen, K.; Zakaria, M.; Huseinovic, N.; Basson, C.T.; et al. Licogliflozin versus Placebo in Women with Polycystic Ovary Syndrome: A Randomized, Double-blind, Phase 2 Trial. Diabetes Obes. Metab. 2021, 23, 2595–2599.
- He, Y.; Haynes, W.; Meyers, C.D.; Amer, A.; Zhang, Y.; Mahling, P.; Mendonza, A.E.; Ma, S.; Chutkow, W.; Bachman, E. The Effects of Licogliflozin, a Dual SGLT1/2 Inhibitor, on Body Weight in Obese Patients with or without Diabetes. Diabetes Obes. Metab. 2019, 21, 1311–1321.
- EMA. Canagliflozin Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/invokana (accessed on 1 December 2023).
- Rodbard, H.W.; Peters, A.L.; Slee, A.; Cao, A.; Traina, S.B.; Alba, M. The Effect of Canagliflozin, a Sodium Glucose Cotransporter 2 Inhibitor, on Glycemic End Points Assessed by Continuous Glucose Monitoring and Patient-Reported Outcomes Among People with Type 1 Diabetes. Diabetes Care 2017, 40, 171–180.
- Taieb, V.; Pacou, M.; Schroeder, M.; Nielsen, A.T.; Neslusan, C.; Schubert, A. Bayesian Network Meta-Analysis to Assess the Relative Efficacy and Safety of Canagliflozin in Patients with Type 2 Diabetes Mellitus (T2DM) Inadequately Controlled on Metformin and Sulphonylurea (MET+SU). Value Health 2013, 16, PDB5.
- EMA. Dapagliflozin Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/forxiga (accessed on 25 September 2023).
- Adamson, C.; Docherty, K.F.; Heerspink, H.J.L.; de Boer, R.A.; Damman, K.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Petrie, M.C.; et al. Initial Decline (Dip) in Estimated Glomerular Filtration Rate After Initiation of Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction: Insights From DAPA-HF. Circulation 2022, 146, 438–449.
- NCT05162690; Efficacy of Dapagliflozin in Diabetes Associated Peripheral Neuropathy. Postgraduate Institute of Medical Education and Research: Chandigarh, India, 2021. Available online: https://clinicaltrials.gov/show/NCT05162690 (accessed on 20 October 2023).
- Palandurkar, G.; Kumar, S. Current Status of Dapagliflozin in Congestive Heart Failure. Cureus 2022, 14, e29413.
- Colombo, G.; Casella, R.; Cazzaniga, A.; Casiraghi, C. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. Intern. Emerg. Med. 2020, 15, 515–517.
- EMA. Empagliflozin Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/jardiance (accessed on 20 November 2023).
- Bteich, F.; Daher, G.; Kapoor, A.; Charbek, E.; Kamel, G. Post-Surgical Euglycemic Diabetic Ketoacidosis in a Patient on Empagliflozin in the Intensive Care Unit. Cureus 2019, 11, e4496.
- Jigheh, Z.A.; Haghjo, A.G.; Argani, H.; Roshangar, L.; Rashtchizadeh, N.; Sanajou, D.; Ahmad, S.N.S.; Rashedi, J.; Dastmalchi, S.; Abbasi, M.M. Empagliflozin Alleviates Renal Inflammation and Oxidative Stress in Streptozotocin-Induced Diabetic Rats Partly by Repressing HMGB1-TLR4 Receptor Axis. Iran. J. Basic Med. Sci. 2019, 22, 384–390.
- Baer, P.C.; Koch, B.; Freitag, J.; Schubert, R.; Geiger, H. No Cytotoxic and Inflammatory Effects of Empagliflozin and Dapagliflozin on Primary Renal Proximal Tubular Epithelial Cells under Diabetic Conditions In Vitro. Int. J. Mol. Sci. 2020, 21, 391.
- Zhang, F.; Wang, W.; Hou, X. Effectiveness and Safety of Ertugliflozin for Type 2 Diabetes: A Meta-analysis of Data from Randomized Controlled Trials. J. Diabetes Investig. 2022, 13, 478–488.
- EMA. Ertugliflozin Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/steglatro-epar-product-information_en.pdf (accessed on 25 November 2023).
- Hoy, S.M. Bexagliflozin: First Approval. Drugs 2023, 83, 447–453.
- Hadd, M.J.; Bienhoff, S.E.; Little, S.E.; Geller, S.; Ogne-Stevenson, J.; Dupree, T.J.; Scott-Moncrieff, J.C. Safety and Effectiveness of the Sodium-glucose Cotransporter Inhibitor Bexagliflozin in Cats Newly Diagnosed with Diabetes Mellitus. J. Vet. Intern. Med. 2023, 37, 915–924.
- Pasqualotto, E.; Figueiredo Watanabe, J.M.; Gewehr, D.M.; da Silva Maintinguer, R.; van de Sande-Lee, S.; de Araujo, G.N.; Leal, F.S.; Pinheiro, C.E.A. Efficacy and Safety of Bexagliflozin in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-analysis. Diabetes Obes. Metab. 2023, 25, 1794–1802.
- Halvorsen, Y.; Walford, G.; Thurber, T.; Russell, H.; Massaro, M.; Freeman, M.W. A 12-week, Randomized, Double-blind, Placebo-controlled, Four-arm Dose-finding Phase 2 Study Evaluating Bexagliflozin as Monotherapy for Adults with Type 2 Diabetes. Diabetes Obes. Metab. 2020, 22, 566–573.
- Halvorsen, Y.; Conery, A.L.; Lock, J.P.; Zhou, W.; Freeman, M.W. Bexagliflozin as an Adjunct to Metformin for the Treatment of Type 2 Diabetes in Adults: A 24-week, Randomized, Double-blind, Placebo-controlled Trial. Diabetes Obes. Metab. 2023, 25, 2954–2962.
- Halvorsen, Y.; Lock, J.P.; Zhou, W.; Zhu, F.; Freeman, M.W. A 24-week, Randomized, Double-blind, Active-controlled Clinical Trial Comparing Bexagliflozin with Sitagliptin as an Adjunct to Metformin for the Treatment of Type 2 Diabetes in Adults. Diabetes Obes. Metab. 2019, 21, 2248–2256.
- Tanaka, M.; Yamakage, H.; Inoue, T.; Odori, S.; Kusakabe, T.; Shimatsu, A.; Satoh-Asahara, N. Beneficial Effects of Ipragliflozin on the Renal Function and Serum Uric Acid Levels in Japanese Patients with Type 2 Diabetes: A Randomized, 12-Week, Open-Label, Active-Controlled Trial. Intern. Med. 2020, 59, 601–609.
- Takasu, T.; Yokono, M.; Tahara, A.; Takakura, S. In Vitro Pharmacological Profile of Ipragliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor. Biol. Pharm. Bull. 2019, 42, 507–511.
- Morishita, A.; Tadokoro, T.; Fujihara, S.; Iwama, H.; Oura, K.; Fujita, K.; Tani, J.; Takuma, K.; Nakahara, M.; Shi, T.; et al. Ipragliflozin Attenuates Non-Alcoholic Steatohepatitis Development in an Animal Model. PLoS ONE 2022, 17, e0261310.
- Yamauchi, Y.; Nakamura, A.; Takahashi, K.; Takase, T.; Yamamoto, C.; Yokota, I.; Atsumi, T.; Miyoshi, H. Factors with Remission of Fatty Liver in Patients with Type 2 Diabetes Treated with Ipragliflozin. Endocr. J. 2019, 66, 995–1000.
- Kashiwagi, A.; Shestakova, M.V.; Ito, Y.; Noguchi, M.; Wilpshaar, W.; Yoshida, S.; Wilding, J.P.H. Safety of Ipragliflozin in Patients with Type 2 Diabetes Mellitus: Pooled Analysis of Phase II/III/IV Clinical Trials. Diabetes Therapy 2019, 10, 2201–2217.
- Bando, S.; Ichikawa, R.; Taguchi, T.; Fujimoto, K.; Motomiya, T.; Taguchi, M.; Takano, K.; Shichiri, M.; Miyatsuka, T. Effects of Luseogliflozin on the Secretion of Islet Hormones and Incretins in Patients with Type 2 Diabetes. Endocr. J. 2022, 69, 681–687.
- Ejiri, K.; Miyoshi, T.; Kihara, H.; Hata, Y.; Nagano, T.; Takaishi, A.; Toda, H.; Nanba, S.; Nakamura, Y.; Akagi, S.; et al. Effect of Luseogliflozin on Heart Failure with Preserved Ejection Fraction in Patients With Diabetes Mellitus. J. Am. Heart Assoc. 2020, 9, e015103.
- Nakashima, M.; Miyoshi, T.; Ejiri, K.; Kihara, H.; Hata, Y.; Nagano, T.; Takaishi, A.; Toda, H.; Nanba, S.; Nakamura, Y.; et al. Effects of Luseogliflozin on Estimated Plasma Volume in Patients with Heart Failure with Preserved Ejection Fraction. ESC Heart Fail. 2022, 9, 712–720.
- Kario, K.; Okada, K.; Murata, M.; Suzuki, D.; Yamagiwa, K.; Abe, Y.; Usui, I.; Tsuchiya, N.; Iwashita, C.; Harada, N.; et al. Effects of Luseogliflozin on Arterial Properties in Patients with Type 2 Diabetes Mellitus: The Multicenter, Exploratory LUSCAR Study. J. Clin. Hypertens. 2020, 22, 1585–1593.
- Osaka, N.; Mori, Y.; Terasaki, M.; Hiromura, M.; Saito, T.; Yashima, H.; Shiraga, Y.; Kawakami, R.; Ohara, M.; Fukui, T.; et al. Luseogliflozin Inhibits High Glucose-Induced TGF- β 2 Expression in Mouse Cardiomyocytes by Suppressing NHE-1 Activity. J. Int. Med. Res. 2022, 50, 030006052210974.
- Chino, Y.; Kuwabara, M.; Hisatome, I. Factors Influencing Change in Serum Uric Acid After Administration of the Sodium-Glucose Cotransporter 2 Inhibitor Luseogliflozin in Patients with Type 2 Diabetes Mellitus. J. Clin. Pharmacol. 2022, 62, 366–375.
- Katakami, N.; Mita, T.; Yoshii, H.; Shiraiwa, T.; Yasuda, T.; Okada, Y.; Torimoto, K.; Umayahara, Y.; Kaneto, H.; Osonoi, T.; et al. Effect of Tofogliflozin on Arterial Stiffness in Patients with Type 2 Diabetes: Prespecified Sub-Analysis of the Prospective, Randomized, Open-Label, Parallel-Group Comparative UTOPIA Trial. Cardiovasc. Diabetol. 2021, 20, 4.
- Kawaguchi, Y.; Sawa, J.; Kumeda, Y. Efficacy and Safety of Tofogliflozin and Ipragliflozin for Patients with Type-2 Diabetes: A Randomized Crossover Study by Flash Glucose Monitoring. Diabetes Ther. 2020, 11, 2945–2958.
- Utsunomiya, K.; Kakiuchi, S.; Senda, M.; Fujii, S.; Kurihara, Y.; Gunji, R.; Koshida, R.; Kameda, H.; Tamura, M.; Kaku, K. Safety and Effectiveness of Tofogliflozin in Japanese Patients with Type 2 Diabetes Mellitus: Results of 24-month Interim Analysis of a Long-term Post-marketing Study (J-STEP/LT). J. Diabetes Investig. 2020, 11, 906–916.
- Markham, A. Remogliflozin Etabonate: First Global Approval. Drugs 2019, 79, 1157–1161.
- Napolitano, A.; Miller, S.; Murgatroyd, P.R.; Hussey, E.; Dobbins, R.L.; Bullmore, E.T.; Nunez, D.J.R. Exploring Glycosuria as a Mechanism for Weight and Fat Mass Reduction. A Pilot Study with Remogliflozin Etabonate and Sergliflozin Etabonate in Healthy Obese Subjects. J. Clin. Transl. Endocrinol. 2014, 1, e3–e8.
- Ding, L.; Liu, S.; Yan, H.; Li, Z.; Zhou, Y.; Pang, H.; Lu, R.; Zhang, W.; Che, M.; Wang, L.; et al. Pharmacokinetics of Henagliflozin in Dialysis Patients with Diabetes. Clin. Pharmacokinet. 2023, 62, 1581–1587.
- Chen, Z.; Li, L.; Zhan, Y.; Zhang, Y.; Liu, H.; Zou, J.; Zhong, D. Characterization and Quantitative Determination of Henagliflozin Metabolites in Humans. J. Pharm. Biomed. Anal. 2021, 192, 113632.
- EMA. Zynquista, Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/zynquista (accessed on 30 November 2023).
- Pérez, M.S.; Rodríguez-Capitán, J.; Requena-Ibáñez, J.A.; Santos-Gallego, C.G.; Urooj Zafar, M.; Escolar, G.; Mancini, D.; Mitter, S.; Lam, D.; Contreras, J.P.; et al. Rationale and Design of the SOTA-P-CARDIA Trial (ATRU-V): Sotagliflozin in HFpEF Patients Without Diabetes. Cardiovasc. Drugs Ther. 2023.
- Markham, A.; Keam, S.J. Sotagliflozin: First Global Approval. Drugs 2019, 79, 1023–1029.
- Cefalo, C.M.A.; Cinti, F.; Moffa, S.; Impronta, F.; Sorice, G.P.; Mezza, T.; Pontecorvi, A.; Giaccari, A. Sotagliflozin, the First Dual SGLT Inhibitor: Current Outlook and Perspectives. Cardiovasc. Diabetol. 2019, 18, 20.
- Sands, A.T.; Zambrowicz, B.P.; Rosenstock, J.; Lapuerta, P.; Bode, B.W.; Garg, S.K.; Buse, J.B.; Banks, P.; Heptulla, R.; Rendell, M.; et al. Sotagliflozin, a Dual SGLT1 and SGLT2 Inhibitor, as Adjunct Therapy to Insulin in Type 1 Diabetes. Diabetes Care 2015, 38, 1181–1188.
- Sotagliflozin (Inpefa) for Heart Failure. Med. Lett. Drugs Ther. 2023, 65, 114–116.
- Davies, M.J.; Sun, F.; Banks, P.; Bhatt, D.L.; Pitt, B. Major Adverse Cardiovascular Events Across the Sotagliflozin Clinical Development Program. Am. Heart J. 2022, 254, 243–244.
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128.
- Sims, H.; Smith, K.H.; Bramlage, P.; Minguet, J. Sotagliflozin: A Dual Sodium-Glucose Co-Transporter-1 and -2 Inhibitor for the Management of Type 1 and Type 2 Diabetes Mellitus. Diabet. Med. 2018, 35, 1037–1048.
- Wang-Lakshman, L.; Mendonza, A.E.; Huber, R.; Walles, M.; He, Y.; Jarugula, V. Pharmacokinetics, Metabolism, and Excretion of Licogliflozin, a Dual Inhibitor of SGLT1/2, in Rats, Dogs, and Humans. Xenobiotica 2021, 51, 413–426.
- Feder, D.; de Fatima Veiga Gouveia, M.R.; Govato, T.C.P.; Nassis, C.D.Z. SGLT2 Inhibitors and the Mechanisms Involved in Weight Loss. Curr. Pharmacol. Rep. 2020, 6, 346–353.
- Kaur, P.; Kotru, S.; Tuteja, L.; Ludhiadch, A.; Munshi, A. Role of SGLT2 Inhibitors in Diabetes Management: Focus on HbA1c Levels, Weight Loss and Genetic Variation. J. Med. Health Stud. 2023, 4, 187–196.
- Giaccari, A. Sodium-glucose Co-transporter Inhibitors: Medications That Mimic Fasting for Cardiovascular Prevention. Diabetes Obes. Metab. 2019, 21, 2211–2218.
- Janež, A.; Fioretto, P. SGLT2 Inhibitors and the Clinical Implications of Associated Weight Loss in Type 2 Diabetes: A Narrative Review. Diabetes Ther. 2021, 12, 2249–2261.
- Vallon, V.; Verma, S. Effects of SGLT2 Inhibitors on Kidney and Cardiovascular Function. Annu. Rev. Physiol. 2021, 83, 503–528.
- Pereira, M.J.; Eriksson, J.W. Emerging Role of SGLT-2 Inhibitors for the Treatment of Obesity. Drugs 2019, 79, 219–230.
- Vaziri, Z.; Saleki, K.; Aram, C.; Alijanizadeh, P.; Pourahmad, R.; Azadmehr, A.; Ziaei, N. Empagliflozin Treatment of Cardiotoxicity: A Comprehensive Review of Clinical, Immunobiological, Neuroimmune, and Therapeutic Implications. Biomed. Pharmacother. 2023, 168, 115686.
- Somagutta, M.K.R.; Luvsannyam, E.; Jain, M.; Cuddapah, G.V.; Pelluru, S.; Mustafa, N.; Nasereldin, D.S.; Pendyala, S.K.; Jarapala, N.; Padamati, B. Sodium Glucose Co-Transport 2 Inhibitors for Gout Treatment. Discoveries 2022, 10, e152.
- Dharia, A.; Khan, A.; Sridhar, V.S.; Cherney, D.Z.I. SGLT2 Inhibitors: The Sweet Success for Kidneys. Annu. Rev. Med. 2023, 74, 369–384.
- Kalay, Z.; Sahin, O.E.; Copur, S.; Danacı, S.; Ortiz, A.; Yau, K.; Cherney, D.Z.I.; Kanbay, M. SGLT-2 Inhibitors in Nephrotic-Range Proteinuria: Emerging Clinical Evidence. Clin. Kidney J. 2023, 16, 52–60.
- Hahn, K.; Ejaz, A.A.; Kanbay, M.; Lanaspa, M.A.; Johnson, R.J. Acute Kidney Injury from SGLT2 Inhibitors: Potential Mechanisms. Nat. Rev. Nephrol. 2016, 12, 711–712.
- Copur, S.; Yildiz, A.; Basile, C.; Tuttle, K.R.; Kanbay, M. Is There Any Robust Evidence Showing That SGLT2 Inhibitor Use Predisposes to Acute Kidney Injury? J. Nephrol. 2022, 36, 31–43.
- Halimi, S.; Vergès, B. Adverse Effects and Safety of SGLT-2 Inhibitors. Diabetes Metab. 2014, 40, S28–S34.
- Qiu, M.; Ding, L.-L.; Zhang, M.; Zhou, H.-R. Safety of Four SGLT2 Inhibitors in Three Chronic Diseases: A Meta-Analysis of Large Randomized Trials of SGLT2 Inhibitors. Diab Vasc. Dis. Res. 2021, 18, 147916412110110.
- Kao, C.-T.; Lee, Y.J.; Al-Battah, H. Eugylcemic Diabetic Ketoacidosis: An Atypical Presentation of Latent Autoimmune Diabetes in Adults (LADA) with Concurrent SGLT2-Inhibitor Use. In Proceedings of the C44. Critical Care Case Reports: Metabolic, Renal, And Endocrine, Philadelphia, PA, USA, 15–20 May 2020; American Thoracic Society International Conference: New York, NY, USA, 2020; p. A5167.
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The Burden and Risks of Emerging Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539.
- Zhang, Q.; Wu, Y.; Lu, Y.; Fei, X. Efficacy and Safety of Metformin and Sodium-Glucose Co-Transporter-2 Inhibitors in Adults with Type 1 Diabetes: A Systematic Review and Network Meta-Analysis. Rev. Clínica Española 2020, 220, 8–21.
- Şahin, S.; Haliloğlu, Ö.; Polat Korkmaz, Ö.; Durcan, E.; Rekali Şahin, H.; Yumuk, V.D.; Damci, T.; Ilkova, H.; Oşar Siva, Z. Does Treatment with Sodium-Glucose Co-Transporter-2 Inhibitors Have an Effect on Sleep Quality, Quality of Life, and Anxiety Levels in People with Type 2 Diabetes Mellitus? Turk. J. Med. Sci. 2021, 51, 735–742.
- Pinto, L.C.; Rados, D.V.; Remonti, L.R.; Kramer, C.K.; Leitao, C.B.; Gross, J.L. Efficacy of SGLT2 Inhibitors in Glycemic Control, Weight Loss and Blood Pressure Reduction: A Systematic Review and Meta-Analysis. Diabetol. Metab. Syndr. 2015, 7, A58.
- Santulli, G.; Varzideh, F.; Forzano, I.; Wilson, S.; Salemme, L.; De Donato, A.; Lombardi, A.; Rainone, A.; Nunziata, L.; Jankauskas, S.S.; et al. Functional and Clinical Importance of SGLT2-Inhibitors in Frailty: From the Kidney to the Heart. Hypertension 2023, 80, 1800–1809.
- Khan, T.; Khan, S.; Akhtar, M.; Ali, J.; Najmi, A.K. Empagliflozin Nanoparticles Attenuates Type2 Diabetes Induced Cognitive Impairment via Oxidative Stress and Inflammatory Pathway in High Fructose Diet Induced Hyperglycemic Mice. Neurochem. Int. 2021, 150, 105158.
- Chen, X.; Zhao, J.; Chen, S. Advances in SGLT2 Inhibitor Research on Cognitive Impairment in Type 2 Diabetes. J. Contemp. Med. Pract. 2022, 4, 37.
- Wang, S.; Fan, F. Abstract 051: Oral Antihyperglycemic Therapy with a SGLT2 Inhibitor Reverses Cognitive Impairments in Elderly Diabetics. Hypertension 2019, 74, 051.
- Pawlos, A.; Broncel, M.; Woźniak, E.; Gorzelak-Pabiś, P. Neuroprotective Effect of SGLT2 Inhibitors. Molecules 2021, 26, 7213.
- Mone, P.; Lombardi, A.; Gambardella, J.; Pansini, A.; Macina, G.; Morgante, M.; Frullone, S.; Santulli, G. Empagliflozin Improves Cognitive Impairment in Frail Older Adults with Type 2 Diabetes and Heart Failure With Preserved Ejection Fraction. Diabetes Care 2022, 45, 1247–1251.
- Lato, M.; Iberszer, K.; Litwiniuk, M.; Zaniuk, M.; Hurkała, K.; Antonik, D.; Denys, B.; Góra, K.; Zimnicki, P.; Zdziennicki, W. Use of SGLT2 Inhibitors in the Treatment of Cognitive Disorders. J. Educ. Health Sport 2023, 25, 27–39.
- Sim, A.Y.; Barua, S.; Kim, J.Y.; Lee, Y.; Lee, J.E. Role of DPP-4 and SGLT2 Inhibitors Connected to Alzheimer Disease in Type 2 Diabetes Mellitus. Front. Neurosci. 2021, 15, 708547.
- NCT04304261; Effects of SGLT2i on the Cognitive Function in T2DM Patient (ESCDP). Third Military Medical University: Chongqing, China, 2020. Available online: https://clinicaltrials.gov/show/NCT04304261 (accessed on 30 October 2023).
- Tang, H.; Shao, H.; Shaaban, C.E.; Yang, K.; Brown, J.; Anton, S.; Wu, Y.; Bress, A.; Donahoo, W.T.; DeKosky, S.T.; et al. Newer glucose-lowering Drugs and Risk of Dementia: A Systematic Review and meta-analysis of Observational Studies. J. Am. Geriatr. Soc. 2023, 71, 2096–2106.
- Panchal, S.; Chhabra, S.; Prasad, B.K.; Aich, B.; Rajani, A. Management of Cognitive Decline in T2DM—SGLT2 Inhibitors at Horizon. Indian J. Endocrinol. Metab. 2018, 22, 20406223221086996.
- Cardoso, R.; Graffunder, F.P.; Ternes, C.M.P.; Fernandes, A.; Rocha, A.V.; Fernandes, G.; Bhatt, D.L. SGLT2 Inhibitors Decrease Cardiovascular Death and Heart Failure Hospitalizations in Patients with Heart Failure: A Systematic Review and Meta-Analysis. EClinicalMedicine 2021, 36, 100933.
- Llorens-Cebrià, C.; Molina-Van den Bosch, M.; Vergara, A.; Jacobs-Cachá, C.; Soler, M.J. Antioxidant Roles of SGLT2 Inhibitors in the Kidney. Biomolecules 2022, 12, 143.
- Tsai, K.-F.; Chen, Y.-L.; Chiou, T.T.-Y.; Chu, T.-H.; Li, L.-C.; Ng, H.-Y.; Lee, W.-C.; Lee, C.-T. Emergence of SGLT2 Inhibitors as Powerful Antioxidants in Human Diseases. Antioxidants 2021, 10, 1166.
- Tang, H.; Dai, Q.; Shi, W.; Zhai, S.; Song, Y.; Han, J. SGLT2 Inhibitors and Risk of Cancer in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Diabetologia 2017, 60, 1862–1872.
- Chavda, V.; Vashi, R.; Patel, S. Cerebrovascular Complications of Diabetes: SGLT-2 Inhibitors as a Promising Future Therapeutics. Curr. Drug Targets 2021, 22, 1629–1636.
- Ren, D.; Sun, Y.; Zhang, D.; Li, D.; Liu, Z.; Jin, X.; Wu, H. SGLT2 Promotes Pancreatic Cancer Progression by Activating the Hippo Signaling Pathway via the HnRNPK-YAP1 Axis. Cancer Lett. 2021, 519, 277–288.
- Wu, W.; Zhang, Z.; Jing, D.; Huang, X.; Ren, D.; Shao, Z.; Zhang, Z. SGLT2 Inhibitor Activates the STING/IRF3/IFN-β Pathway and Induces Immune Infiltration in Osteosarcoma. Cell Death Dis. 2022, 13, s11419.
- Luo, J.; Hendryx, M.; Dong, Y. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors and Non-Small Cell Lung Cancer Survival. Br. J. Cancer 2023, 128, 1541–1547.
- Shaikh, A.M.Y. SGLT2 Inhibitors and Cancer: Why Further Evidence Is Required. Diabetologia 2017, 60, 2536–2537.
- Scafoglio, C.; Hirayama, B.A.; Kepe, V.; Liu, J.; Ghezzi, C.; Satyamurthy, N.; Moatamed, N.A.; Huang, J.; Koepsell, H.; Barrio, J.R.; et al. Functional Expression of Sodium-Glucose Transporters in Cancer. Proc. Natl. Acad. Sci. USA 2015, 112, E4111–E4119.
- Wang, Y.; Yang, L.; Mao, L.; Zhang, L.; Zhu, Y.; Xu, Y.; Cheng, Y.; Sun, R.; Zhang, Y.; Ke, J.; et al. SGLT2 Inhibition Restrains Thyroid Cancer Growth via G1/S Phase Transition Arrest and Apoptosis Mediated by DNA Damage Response Signaling Pathways. Cancer Cell Int. 2022, 22, 74.
- Xian-Huan, B. Research Progress on Effect of Sodium-Glucose Cotransporter 2 Inhibitors on Tumor. Drugs Clin. 2022, 36, 21756.
- Abouelkheir, M.; Taha, A.E. SGLT2 Inhibitors and Cancer: Is Immunity the Missing Link? J. Pharmacol. Clin. Res. 2019, 6, 555699.
- Hu, W.-S.; Lin, C.-L. Patients with Diabetes with and without Sodium-Glucose Cotransporter-2 Inhibitors Use with Incident Cancer Risk. J. Diabetes Complicat. 2023, 37, 108468.
- Ali, A.; Mekhaeil, B.; Biziotis, O.-D.; Tsakiridis, E.E.; Ahmadi, E.; Wu, J.; Wang, S.; Singh, K.; Menjolian, G.; Farrell, T.; et al. The SGLT2 Inhibitor Canagliflozin Suppresses Growth and Enhances Prostate Cancer Response to Radiotherapy. Commun. Biol. 2023, 6, 919.
- Bardaweel, S.; Issa, A. Exploring the Role of Sodium-Glucose Cotransporter as a New Target for Cancer Therapy. J. Pharm. Pharm. Sci. 2022, 25, 253–265.
- Hendryx, M.; Dong, Y.; Ndeke, J.M.; Luo, J. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitor Initiation and Hepatocellular Carcinoma Prognosis. PLoS ONE 2022, 17, e0274519.
- Gyimesi, G.; Pujol-Giménez, J.; Kanai, Y.; Hediger, M.A. Sodium-Coupled Glucose Transport, the SLC5 Family, and Therapeutically Relevant Inhibitors: From Molecular Discovery to Clinical Application. Pflug. Arch. 2020, 472, 1177–1206.
- Wu, W.; Wang, Y.; Xie, J.; Fan, S. Empagliflozin: A Potential Anticancer Drug. Discov. Oncol. 2023, 14, 127.
- Komatsu, S.; Nomiyama, T.; Numata, T.; Kawanami, T.; Hamaguchi, Y.; Tanaka, T.; Inoue, R.; Yanase, T. SGLT2 Inhibitor Ipragliflozin Induces Breast Cancer Apoptosis via Membrane Hyperpolarization and Mitochondria Dysfunction. Diabetes 2018, 67, 255-OR.
- Perry, R.J.; Shulman, G.I. Sodium-Glucose Cotransporter-2 Inhibitors: Understanding the Mechanisms for Therapeutic Promise and Persisting Risks. J. Biol. Chem. 2020, 295, 14379–14390.
- Saijo, Y.; Okada, H.; Hata, S.; Nakajima, H.; Kitagawa, N.; Okamura, T.; Osaka, T.; Kitagawa, N.; Majima, S.; Senmaru, T.; et al. Reasons for Discontinuing Treatment with Sodium-Glucose Cotransporter 2 Inhibitors in Patients with Diabetes in Real-World Settings: The KAMOGAWA-A Study. J. Clin. Med. 2023, 12, 6993.




