The primary treatment for autoimmune Diabetes Mellitus (Type 1 Diabetes Mellitus-T1DM) is insulin therapy. Unfortunately, a multitude of clinical cases has demonstrated that the use of insulin as a sole therapeutic intervention fails to address all issues comprehensively. Therefore, non-insulin adjunct treatment has been investigated and shown successful results in clinical trials. Various hypoglycemia-inducing drugs such as Metformin, glucagon-like peptide 1 (GLP-1) receptor agonists, dipeptidyl peptidase-4 (DPP-4) inhibitors, amylin analogs, and Sodium-Glucose Cotransporters 2 (SGLT-2) inhibitors, developed good outcomes in patients with T1DM. Currently, SGLT-2 inhibitors have remarkably improved the treatment of patients with diabetes by preventing cardiovascular events, heart failure hospitalization, and progression of renal disease. However, their pharmacological potential has not been explored enough.
1. Introduction
Due to considerable glycemic and non-glycemic benefits, the FDA approved several selective SGLT-2 inhibitors for T2DM treatment [
35]. Currently, all available gliflozins as drugs commercialized in pharmacies (Canagliflozin, Dapagliflozin, Empagliflozin, Ertugliflozin, and Bexagliflozin) have T2DM as a main common indication. Heart failure (HF) is mentioned for Dapagliflozin and Empagliflozin, and chronic kidney disease (CKD) only for Dapagliflozin.
Sotagliflozin, a dual SGLT-1/2 inhibitor, is the only representative officially indicated in T1DM.
Other new similar compounds (Ipragliflozin, Luseogliflozin, Tofogliflozin, and Remogliflozin) are investigated in clinical studies. The most recent SGLT inhibitors are investigated using pharmacokinetic and pharmacodynamic analyses (Remoglicoflozin [
36,
37,
38,
39], Henaglicoflozin [
40,
41,
42,
43], and Licoglicoflozin [
44,
45,
46,
47]).
All data are summarized in Table 1.
Table 1. FDA-approved/in-trials SGLT-2 Inhibitors for T2DM therapy.
SGLT-2
Inhibitor |
Active
Ingredient(s) |
Daily Dose
(mg) |
Brand Name |
Company |
| Selective SGLT-2 Inhibitors |
Canagliflozin
[48,49,50] |
Canagliflozin |
100 |
Invokana |
Janssen-Cilag
International NV
Beerse, Belgium |
| Canagliflozin + Metformin |
50/500
50/1000
150/500
150/1000 |
Invokamet |
| Canagliflozin + Metformin extended-release |
Invokamet XR |
Dapagliflozin
[51,52,53,54,55] |
Dapagliflozin |
5
10 |
Forxiga |
AstraZeneca AB
Södertälje
Sweden |
Dapagliflozin + Metformin
extended-release |
5/1000
5/850 |
Xigduo XR |
| Dapagliflozin + Saxagliptin |
5/10 |
Qtern |
Empagliflozin
[56,57,58,59] |
Empagliflozin |
10
25 |
Jardiance |
Boehringer Ingelheim International GmbH
Ingelheim am Rhein,
Germany |
| Empagliflozin + Linagliptin |
10/5
25/5 |
Glyxambi |
| Empagliflozin + Metformin |
5/1000
5/850
12.5/1000
12.5/850 |
Synjardy |
Empagliflozin + Metformin
extended-release |
25/1000 |
Synjardy XR |
Ertugliflozin
[60,61] |
Ertugliflozin |
5
15 |
Steglatro |
Merck Sharp & Dohme
Haarlem,
Netherland |
| Ertugliflozin + Metformin |
2.5/850
7.5/850 |
Segluromet |
| Ertugliflozin + Sitagliptin |
5/100
15/100 |
Steglujan |
Bexagliflozin
[62,63,64,65,66,67] |
Bexagliflozin |
20 |
Brenzavvy |
TheracosBio, LLC
Marlborough, MA, USA |
Ipragliflozin
[68,69,70,71,72] |
Ipragliflozin |
25
50 |
Suglat |
Astellas Pharma LTD,
Addlestone, UK |
Luseogliflozin
[73,74,75,76,77,78] |
Luseogliflozin |
2.5
5 |
Lusefi |
Taisho Pharmaceutical Holdings Co., Ltd.,
Tokyo, Japan |
Tofogliflozin
[79,80,81] |
Tofogliflozin |
20
40 |
Apleway |
Chugai Pharmaceutical Co., Ltd.,
Tokyo, Japan |
Remogliflozin
[37,38,39,82,83] |
Remogliflozin |
100 |
Remogliflozin
etabonate |
GlaxoSmithKline plc, Brentford, UK
Glenmark Pharmceuticals Ltd.,
Mumbai, India |
Henagliflozin
[40,41,42,84,85] |
Henagliflozin |
5
10 |
SHR3824 |
Jiangsu Hengrui Pharmaceuticals Co., Ltd.,
Lianyungang,
China |
| Dual SGLT-2 + SGLT-1 Inhibitors |
Sotagliflozin
[86,87,88,89,90,91,92,93,94] |
Sotagliflozin |
200
400 |
Zynquista
Inpefa |
Lexicon
Pharmaceuticals, Inc.,
The Woodlands, TX, USA |
Licogliflozin
[44,45,46,47,95] |
Licogliflozin |
No data |
LIK-066 |
Novartis AG
Basel, Switzerland |
2. Benefits of SGLT-2 Inhibitors
Globally, SGLT-2 inhibitors are approximately the most prescribed oral antidiabetic drugs. Their beneficial effects beyond glycemic control include weight loss, protection against major cardiovascular events, blood pressure reduction, and delaying the progression of chronic kidney disease (CKD).
2.1. Weight Loss
SGLT-2i directly reduces body weight by removing glucose through urine, thus increasing calorie loss. Glycosuria due to the SGLT-2 inhibitors leads to lower plasma glucose and insulin levels, followed by increased fasting and post-meal glucagon concentration. While blood glucose concentration is diminished, lipid storage is mobilized to be used as an energy substrate. The persistent excretion of glucose in urine induces increasing gluconeogenesis, suppresses tissue glucose disposal and glucose oxidation, accelerates lipolysis and fat oxidation, and enhances ketogenesis. The overall result of these metabolic changes resembles a fasting state, which will cause the loss of fat mass and weight in the long run [
96]. Another study shows the concomitance of weight loss and Hemoglobin A1c level diminution during the therapy with SGLT-2is [
97]. Thus, sodium-glucose cotransporter inhibitors could be called mimic-fasting medication to prevent cardiovascular complications [
98,
99]. SGLT-2 inhibitors reduce body weight by around 2–4 kg; this average is constant for all representatives and persists for up to 4 years [
96].
2.2. Heart Protection
Renal function improvement and preservation through hemodynamic and nonhemodynamic mechanisms are essential for SGLT-2 inhibitors in heart protection [
100]. It is necessary to know the interactions between all biochemical processes, the chronology of all changes, and their correlation with the cardiovascular benefits of gliflozin therapy [
101]. The potential mechanisms [
31] are diuresis and natriuresis, changes in myocardial energetics, increased erythropoietin (EPO) production and erythropoiesis, changes in reno-cardiac signaling, inhibition of the sympathetic nervous system, inhibition of the Na
+/H
+ Exchanger-1 (NHE1), inhibition of the NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome, potential vascular effects, and reducing uric acid levels in serum [
102].
2.3. Kidney Protection
The highlight of SGLT-2i kidney protection led to significant interest in gliflozins’ broader applications in CKD therapy [
103]. The potential mechanisms of their protective effects could be glucosuria, inducing natriuresis and osmotic diuresis and leading to reduced plasma volume and lower blood pressure, reducing proteinuria, and delaying CKD progression in patients with albuminuria [
104], hemodynamic changes at the systemic and glomerular levels, metabolic pathway, and decreasing oxidative stress and inflammation.
3. Adverse Effects of SGLT-2 Inhibitors
3.1. Acute Kidney Injury (AKI)
The SGLT-2is induces glucose and sodium overexcretion, conducting osmotic diuresis. It may lead to hyperosmolarity and dehydration, increasing the risk of AKI [
105]. Recent studies rigorously demonstrate that SGLT-2 inhibitors are safe for the kidneys and do not predispose to AKI [
106].
3.2. Polyuria
Significant glucosuria and natriuria induced by SGLT-2is lead to polyuria due to osmotic diuresis.
3.3. Euglycemic Diabetic Ketoacidosis (DKA)
Production of ketone bodies may show a dual face. The heart and brain can rapidly use them as energetic substrates, avoiding the accumulation of fatty acid or glucose metabolites. Normal ketone levels are <0.6 mmol/L. Most patients treated with SGLT-2is concomitantly have ketosis (ketone levels are slightly increased 0.6–1.5 mmol/L), and they do not develop ketoacidosis. Thus, the tendency toward ketosis can be augmented by a low-carb intake in patients who are trying to lose weight. Ketone bodies are produced by the oxidation of fatty acids in the liver as a source of alternative energy, generally occurring in glucose-limiting conditions. Elevated blood levels of acetoacetate (AA), 3-β-hydroxybutyrate (BHB), and acetone are known as hyperketonemia. BHB serum concentration increases after fasting but should not exceed 0.4 mmol/L. High levels of circulating ketones are linked to oxidative stress and numerous morbid conditions.
The risk of DKA is 1.5–3 mmol/L. DKA manifests when the ketone level is highly increased, over 3 mmol/L. Possible mechanisms [
105] of SGLT-2 inhibitors associated with euglycemic DKA could be noninsulin-dependent glucose clearance, hyperglucagonemia, and volume depletion.
3.4. Genito-Urinary Tract Infections
Glycosuria is the main factor implied in these infectious diseases, being a favorable medium for the growth of bacterial and fungal strains. They are more frequent in females and can be easily treated with non-expensive drugs.
3.5. Bone Fractures and Amputation Risk
Potential mechanisms for fractures [
107] could be volume contraction leading to dizziness and falls, possible effects on calcium, phosphate, and vitamin D homeostasis, and reduction in bone mineral density. The amputation risk [
108] is linked to peripheral vascular disease, neuropathy, history of diabetic foot ulcer, and previous history of amputations. Euglycemic DKA and its potential association with a significant risk for lower-extremity amputation represent sporadic but possible fatal adverse events of SGLT-2is. Some studies reported that SGLT-2 inhibitors in T2DM may cause latent autoimmune DM of adulthood (LADA) [
109].
4. SGLT-2 Inhibitors in the Therapy of DM Complications and Comorbidities
Despite the common knowledge that DM is associated with most known complications (traditional complications such as stroke, coronary heart disease, and heart failure, peripheral neuropathy, retinopathy, diabetic kidney disease), an increased prevalence of cardiovascular pathology and a group of lesser-studied ones have been reported (cancer, infections, functional and cognitive disability, liver disease and affective disorders) [
110].
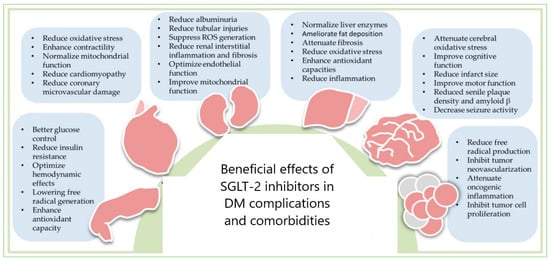
The benefits of SGLT-2 inhibitors in DM complications and comorbidities could be explained through the dual redox behavior of gliflozins (
Figure 1) because oxidative stress has an essential role in their onset and harmful evolution. High blood glucose levels induce ROS production, leading to overexpression of the SGLT-2 in tubular cells, exacerbating oxidative stress. SGLT-2is has demonstrated clear cardiovascular and renal protection due to its antioxidant properties. Severe systemic comorbidity that involves the whole body, leading to functional decline and harmful outcomes [
111,
112,
113] is frailty [
114,
115,
116,
117,
118,
119,
120] or functional disability [
121,
122,
123,
124], and its management is still debated [
119,
120]. SGLT-2i positively acts on cardiovascular complications, especially on the HF rehospitalization rate [
125], during several potential mechanisms: improving cardiovascular energetics, reducing vascular tone and blood pressure, decreasing systemic inflammation, atheroprotective effects, reduction of vascular damage, and direct neuroprotective mechanisms (acetylcholinesterase inhibition and increase in cerebral levels of brain-derived neurotrophic factor). All benefits could be maintained through rigorous balancing between oxidant and antioxidant processes [
126].
Figure 1. SGLT-2 inhibitors’ therapeutic potential in DM complications and comorbidities: cardiovascular diseases, nephropathy, liver diseases, neural disorders, and cancers—reproduction with permission from [
127].
The anticancer potential of gliflozins depends on the blood glucose-lowering capacity [
128,
129]. SGLT-2 inhibitors induce apoptosis and DNA damage, reducing cancer cell proliferation [
130,
131,
132,
133,
134,
135,
136,
137] through mitochondrial membrane instability metabolic changes (oxidative phosphorylation, DNA synthesis, glycolysis, ATP and fatty acids level diminution, beta-oxidation, and ketone amount augmentation).
DM is also associated with cancer development through a complex mechanism. Treatment with SGLT-2 inhibitors can diminish the risk of cancer incidence in DM patients [
138] because gliflozins have anticancer activity through various mechanisms, as previously shown [
139,
140,
141,
142,
143,
144,
145]. Moreover, SGLT-2 inhibitors can protect DM patients against the cardiotoxic action of anticancer drugs [
31].
5. DM Patient Adherence to SGLT-2is Therapy
The most common reasons for discontinuing treatment with SGLT-2is are frequent urination, genital infection, improved glycemic control, and renal dysfunction. A recent retrospective study [
146] did not find a correlation between patients’ compliance and DM type, duration, diabetic control, renal function, or DM complications of diabetes in both groups. Only the age was correlated (the adherence negatively correlates with the patient’s age).
This entry is adapted from the peer-reviewed paper 10.3390/ijms25041972