Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Omar Cauli | -- | 1819 | 2024-02-21 09:43:50 | | | |
| 2 | Lindsay Dong | Meta information modification | 1819 | 2024-02-23 02:25:26 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Brognara, L.; Caballero-Luna, O.; Traina, F.; Cauli, O. Inflammatory Biomarkers and Gait Impairment in Older Adults. Encyclopedia. Available online: https://encyclopedia.pub/entry/55273 (accessed on 08 February 2026).
Brognara L, Caballero-Luna O, Traina F, Cauli O. Inflammatory Biomarkers and Gait Impairment in Older Adults. Encyclopedia. Available at: https://encyclopedia.pub/entry/55273. Accessed February 08, 2026.
Brognara, Lorenzo, Oscar Caballero-Luna, Francesco Traina, Omar Cauli. "Inflammatory Biomarkers and Gait Impairment in Older Adults" Encyclopedia, https://encyclopedia.pub/entry/55273 (accessed February 08, 2026).
Brognara, L., Caballero-Luna, O., Traina, F., & Cauli, O. (2024, February 21). Inflammatory Biomarkers and Gait Impairment in Older Adults. In Encyclopedia. https://encyclopedia.pub/entry/55273
Brognara, Lorenzo, et al. "Inflammatory Biomarkers and Gait Impairment in Older Adults." Encyclopedia. Web. 21 February, 2024.
Copy Citation
Peripheral inflammation and gait speed alterations are common in several neurological disorders and in the aging process, but the association between the two is not well established. Biomarkers play an important role in the decision-making process, and IL-6 can be an effective biomarker in establishing the diagnosis of slow gait speed. Further longitudinal research is needed to establish the use of molecular biomarkers in monitoring gait impairment.
cytokines
molecular marker
falls
IL-6
1. Introduction
Gait velocity is a simple screen of functional status in older adults, and predicts major adverse outcomes in older individuals such as falls, dementia, and death [1][2][3][4][5][6]. A gait speed lower than 0.8 m/s is a reliable cut-off for identifying subjects at increased risk of disability, and evidence on the relationship between circulating IL-6 levels and gait speed suggests that higher IL-6 levels may be associated with poorer performance, hospitalization, institutionalization, and death in older adults [7][8][9][10][11]. Falls are common in older adults, but little is known about accidental falls and their relationship to movement disorders and chronic inflammation. It has been estimated that adults older than 60 years of age suffer the most fatal falls, therefore as the prevalence of older fallers is predicted to increase with changes in demography, prevention strategies should prioritize fall-related research and establish effective strategies to reduce risk [12]. According to the World Health Organization, approximately 684,000 fatal falls occur each year, making it the second leading cause of unintentional injury death after road traffic injuries [13]. The increasing number of people who suffer falls is also an economic problem for health services worldwide, with an estimated cost for the EU of 25 billion Euros for treating fall-related injuries [14]. In addition, people who have experienced a fall develop fear of falling, which is a serious issue that negatively impacts their physical and mental health [15]. Movement disorders and chronic inflammation are frequently comorbid and underdiagnosed [7]. Systemic inflammation is closely associated with central neuroinflammation [8]. The accessibility and practicality of using blood samples have led to numerous studies measuring the profile of serum or plasma immune markers in several neurological disorders, and many of these studies have found a significant association between those markers and disease severity. Other spatiotemporal parameters, such as a stride length of 0.64 m, accurately predict major adverse events such as physical disability, falls, institutionalization, and mortality [16]. In addition, C-reactive protein (CRP) has been significantly and negatively associated with the total number of daily strides [17].
Previous studies of older adults have linked gait and mobility problems to high CRP levels, and chronic inflammation may be a factor affecting denervation of muscles and changes in the neuromuscular junction [18][19]. Inflammation also plays a role in the initiation and progression of knee osteoarthritis, and studies have shown that osteoarthritis is associated with high serum levels of inflammatory markers that alter gait mechanism [20][21][22][23][24][25].
Recent developments in drug therapies with biological agents such as anti-tumor necrosis factor alpha (TNF-α), have provided great benefits in terms of reductions in joint inflammation, pain, and improved gait function [26]. Levels of pro-inflammatory cytokines, and in particular, elevated IL-6 and CRP have been identified as independent predictors of impaired mobility, disability, and slow walking speed in older adults [7][19][27][28][29][30][31][32][33][34][35].
There is growing evidence of associations between elevated levels of inflammatory cytokines such as IL-6, TNF-α, or the acute phase CRP and several chronic health conditions or adverse aging outcomes including muscle loss and cognitive impairment (Figure 1) [34][35].
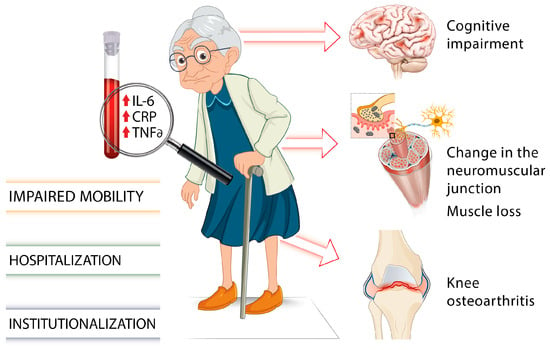
Figure 1. Involvement of proinflammatory markers on gait impairments and frailty in older individuals [7][18][19][20][21][22][23][24][25][26][27][28][29][30][31][32][33][34][35].
2. Inflammatory Biomarkers and Gait Impairment in Older Adults
2.1. CRP
CRP as an inflammatory marker predictive of decreased gait was the most frequently used study parameter, and was included in 13 of the 21 articles analyzed. High sensitivity CRP (hs-CRP) was used for the relationship with gait parameters in five of them [36][37][38][39][40].
In one study [41], several groups were categorized according to their initial CRP, taking 1.33 mg/dL as a low value, and 2.7 mg/dL as a high value. Values higher than 2.7 mg/dL were associated with a greater probability of subsequent disability. They were also associated with the following: activities of daily living (ADL), instrumental activities of daily living (IADL), impaired balance, impaired walking speed, and arthritis.
Elevated CRP levels were related to low vitamin D levels [42], with a higher probability of slow walking speed, which was attenuated when vitamin D deficiency was absent, although the relevant factor inducing slow gait was CRP. Another study [43] investigated major mobility impairment (MMD) and reported consistent relationships with elevated CRP (>3.0 mg/dL) and a higher risk of MMD, which increased the risk by 38% with a sensitivity of 63% and specificity of 54% as a predictive value over 5 years.
The relationship between CRP and fibrinogen [44] with gait speed was studied with 373 people over 20 years, associating the two parameters with gait speed as a single task after adjusting for cardiovascular and risk parameters. However, when executive functions were taken into account, the association with CRP disappeared and the association with fibrinogen was attenuated. Dupont et al. [39] used the combination of two parameters, white blood cell count and high-sensitivity CRP, as factors related to the onset of sarcopenia, and observed a negative association with baseline physical activity, quality of life scores, and the SF-36 physical component score in middle-aged and older men. Baseline leukocyte levels were negatively associated with gait velocity, isometric 90° quadriceps and isokinetic peak torque. In another study with 421 participants [45], where CRP was related to motor cognitive risk syndrome (MCR), characterized by cognitive complaints and slow gait, patients with higher CRP levels (2.8 mg/dL) had a higher probability of MCR if memory impairment was present.
2.2. Relationship of CRP with Other Biomarkers
Langmann et al. [40] related several inflammatory markers, i.e., hs-CRP, TNFα, and its two receptors (TNFα-R1 and TNFα-R2), interleukin-6 (IL-6), soluble IL-6 receptor (sIL-6R), and interleukin-10 (IL-10) with measures of frailty, function, mobility, and falls in elderly women, and observed that frail patients had significantly higher levels of hs-CRP, TNFα-R1, TNFα-R2, IL-6, and IL-6-sR. Higher baseline levels of hs-CRP and IL-6 were associated with worse physical performance and gait speed at 12 months.
A study of patients with Parkinson’s disease determined hs-CRP levels in patients with freezing of gait (FOG). Patients with FOG showed higher levels of hs-CRP than those without FOG, establishing a FOG-No FOG cut-off level of 0.935 mg/dL, with a sensitivity of 87.1% and specificity of 89.2%.
Dupont et al. [46] also analyzed IL-6, IL-8, IL-1β, CRP, and TNFα in sarcopenic subjects. Proinflammatory IL-1β correlated positively with manual grip strength and IL-6 with myofascial autoliberation (aLM). IL-6 correlated inversely with step count. IL-8 was inversely correlated with manual grip strength in women but not in men. In contrast, the proinflammatory cytokines CRP, IL-6, and TNFα correlated inversely with the physical component score of the SF-36 in men, but not in women.
2.3. IL-6 and TNF-Alpha
Brown [47] first reported that individuals who had a high IL-6 concentration in blood (3.2 pg/mL) together with previous slow gait and depression, were associated with slow gait. Older individuals in the highest blood IL-6 quartile (more than 4.60 pg/mL) had a 1.75 cm/s/year faster decline in gait speed compared to the participants with IL-6 levels in the lowest quartile [48]. Custodero [49] evaluated the effects of physical performance intervention on 400-m gait speed at 12-month follow-up according to annual IL-6 change categorized by a 1 pg/mL increase or decrease, and observed that subjects with an annual IL-6 change of between −1 and +2 pg/mL had a significant difference in gait speed in the PA intervention group compared with the healthy educational intervention group. In a study of 333 participants with a mean follow-up of 2.3 years, Verghese et al. [7] found that each one-unit increase in log IL-6 levels was associated with a 0.98/cm/s/year increase in the rate of gait velocity decline. Participants in the highest quartile of IL-6 (4.60 pg/mL) had significantly slower gait velocity compared to the other participants. In another study [50], IL-6 levels were positively correlated with knee extensor and knee flexor power. The final model showed that the factors of plasma IL-6 concentrations and physical activity level explained 4.1% of the mean knee flexor power variability, and plasma IL-6 concentration was the variable that best explained the isokinetic variable. Interestingly, participants in the highest tertile of IL-6 (IL-6 > 2.51 pg/mL) were 1.76 times more likely to develop at least mobility-disability and 1.62 times more likely to develop mobility plus ADL-disability, compared with the lowest percentage of IL-6 (1.75 pg/mL) [31].
2.4. Relationship between IL-6 and TNF-Alpha and Other Biomarkers
The association between IL-6 and major mobility disability (MMD) was investigated [43] and revealed that elevated levels above 2.5 pg/mL were associated with an increased risk of MMD compared to low values ≤2.5 pg/mL. In combination with elevated CRP (>3.0 mg/dLL) alone, patients with both elevated CRP and IL-6 had a 37% increased risk of MMD with a sensitivity of 75% and specificity of 34%.
3. Summary
Inflammation has adverse affective, cognitive, motor, and neurostructural consequences for older adults [47][51]. In older people, levels of IL-6 higher than 2.5 pg/mL predict a future risk of gait speed decline and a higher risk of functional decline over the subsequent 4 years [7][52]. Elevated sensitive CRP concentrations (≥3 mg/L) at baseline were associated with a faster annual decline in gait velocity of 0.91 cm/s [41] and an increased likelihood of motoric cognitive risk syndrome [45]. In terms of cognitive-motor syndrome, physical function and cognitive function share common neurological processes, and a cognitive decline in tandem with a loss of muscle strength places elderly people at increased risk of personal injury, poor mobility, fall-related injuries leading to frailty, reduced independence, and poorer quality of life [48][53][54][55][56]. In fact, older adults show a high prevalence of gait disorders, and higher IL-6 is linked to a larger volume of white matter hyperintensities (in MRI) that are correlated to slow gait [52][57][58][59][60][61]. Inflammation is common both in aging and frailty, while chronic inflammation is associated with decreased muscle mass and strength, disability, dementia, increased morbidity, and mortality. Studies on the impact of exercise on proinflammatory and anti-inflammatory cytokines have shown that physical exercise in pre-frail older adults in primary care improved depression, gait speed, muscle mass indices, physical function, frailty, and had significant improvement of TNFα levels at 3 months [62][63]. An increase in hs-CRP levels was negatively associated with gait speed only in older adults, but not in middle-aged men [39]. It seems reasonable that over time, chronic inflammation might be a factor affecting denervation of muscles and changes in the neuromuscular junction [18][19][64]. Inflammation also plays a role in the initiation and progression of knee osteoarthritis, and several studies have shown that osteoarthritis is associated with high serum levels of inflammatory markers that alter gait mechanisms [20][21][22][23][24][25].
Substantial evidence suggests that the peripheral inflammatory biomarkers IL-6 and CRP are related to frailty status and gait impairment. Cytokines are key chemical mediators of the immune response produced by cells, and an understanding of the molecular, biomechanical, and neuropsychological networks involved is required in order to enhance prevention strategies of gait impairments and its complications, such as falls among the aging population.
References
- Montero-Odasso, M.; Schapira, M.; Soriano, E.R.; Varela, M.; Kaplan, R.; Camera, L.A.; Mayorga, L.M. Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1304–1309.
- Abellan Van Kan, G.; Rolland, Y.; Andrieu, S.; Bauer, J.; Beauchet, O.; Bonnefoy, M.; Cesari, M.; Donini, L.; Gillette-Guyonnet, S.; Inzitari, M.; et al. Gait speed at usual pace as a predictor of adverse outcomes in community-dwelling older people an International Academy on Nutrition and Aging (IANA) Task Force. J. Nutr. Health Aging 2009, 13, 881–889.
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 896–901.
- Verghese, J.; LeValley, A.; Hall, C.B.; Katz, M.J.; Ambrose, A.F.; Lipton, R.B. Epidemiology of Gait Disorders in Community-Residing Older Adults. J. Am. Geriatr. Soc. 2006, 54, 255–261.
- Ruiz-Ruiz, L.; Jimenez, A.R.; Garcia-Villamil, G.; Seco, F. Detecting Fall Risk and Frailty in Elders with Inertial Motion Sensors: A Survey of Significant Gait Parameters. Sensors 2021, 21, 6918.
- Studenski, S. Gait Speed and Survival in Older Adults. JAMA 2011, 305, 50.
- Verghese, J.; Holtzer, R.; Oh-Park, M.; Derby, C.A.; Lipton, R.B.; Wang, C. Inflammatory Markers and Gait Speed Decline in Older Adults. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66A, 1083–1089.
- Hardy, S.E.; Perera, S.; Roumani, Y.F.; Chandler, J.M.; Studenski, S.A. Improvement in Usual Gait Speed Predicts Better Survival in Older Adults. J. Am. Geriatr. Soc. 2007, 55, 1727–1734.
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31.
- Pamoukdjian, F.; Lévy, V.; Sebbane, G.; Boubaya, M.; Landre, T.; Bloch-Queyrat, C.; Paillaud, E.; Zelek, L. Slow gait speed is an independent predictor of early death in older cancer outpatients: Results from a prospective cohort study. J. Nutr. Health Aging 2017, 21, 202–206.
- Zhu, S.; Patel, K.V.; Bandinelli, S.; Ferrucci, L.; Guralnik, J.M. Predictors of Interleukin-6 Elevation in Older Adults. J. Am. Geriatr. Soc. 2009, 57, 1672–1677.
- Hacıdursunoğlu Erbaş, D.; Çınar, F.; Eti Aslan, F. Elderly patients and falls: A systematic review and meta-analysis. Aging Clin. Exp. Res. 2021, 33, 2953–2966.
- World Health Organization. Falls. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/falls (accessed on 20 July 2023).
- Almada, M.; Brochado, P.; Portela, D.; Midão, L.; Costa, E. Prevalence of falls and associated factors among community-dwelling older adults: A cross-sectional study. J. Frailty Aging 2021, 10, 10–16.
- Gambaro, E.; Gramaglia, C.; Azzolina, D.; Campani, D.; Molin, A.D.; Zeppegno, P. The complex associations between late life depression, fear of falling and risk of falls. A systematic review and meta-analysis. Ageing Res. Rev. 2022, 73, 101532.
- Bytyçi, I.; Henein, M.Y. Stride Length Predicts Adverse Clinical Events in Older Adults: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 2670.
- Gardner, A.W.; Parker, D.E.; Montgomery, P.S.; Blevins, S.M.; Teague, A.M.; Casanegra, A.I. Monitored Daily Ambulatory Activity, Inflammation, and Oxidative Stress in Patients with Claudication. Angiology 2014, 65, 491–496.
- Gonzalez-Freire, M.; de Cabo, R.; Studenski, S.A.; Ferrucci, L. The Neuromuscular Junction: Aging at the Crossroad between Nerves and Muscle. Front. Aging Neurosci. 2014, 6, 208.
- Sousa, A.C.P.A.; Zunzunegui, M.-V.; Li, A.; Phillips, S.P.; Guralnik, J.M.; Guerra, R.O. Association between C-reactive protein and physical performance in older populations: Results from the International Mobility in Aging Study (IMIAS). Age Ageing 2016, 45, 274–280.
- Edd, S.N.; Favre, J.; Blazek, K.; Omoumi, P.; Asay, J.L.; Andriacchi, T.P. Altered gait mechanics and elevated serum pro-inflammatory cytokines in asymptomatic patients with MRI evidence of knee cartilage loss. Osteoarthr. Cartil. 2017, 25, 899–906.
- Mabey, T. Cytokines as biochemical markers for knee osteoarthritis. World J. Orthop. 2015, 6, 95.
- Shultz, S.P.; Buck, A.N.; Fink, P.W.; Kung, S.M.; Ward, M.J.; Antal, Z.; Backus, S.I.; Kraszewski, A.P.; Hillstrom, H.J. Body mass affects kinetic symmetry and inflammatory markers in adolescent knees during gait. Clin. Biomech. 2023, 102, 105887.
- Addison, O.; Drummond, M.J.; Lastayo, P.C.; Dibble, L.E.; Wende, A.R.; McClain, D.A.; Marcus, R.L. Intramuscular fat and inflammation differ in older adults: The impact of frailty and inactivity. J. Nutr. Health Aging 2014, 18, 532–538.
- Bautmans, I.; Njemini, R.; Predom, H.; Lemper, J.-C.; Mets, T. Muscle Endurance in Elderly Nursing Home Residents Is Related to Fatigue Perception, Mobility, and Circulating Tumor Necrosis Factor-Alpha, Interleukin-6, and Heat Shock Protein 70. J. Am. Geriatr. Soc. 2008, 56, 389–396.
- Broström, E.; Esbjörnsson, A.-C.; von Heideken, J.; Larsson, P.; Wretenberg, P.; Iversen, M. Change in Gait Deviation Index after anti-tumour necrosis factor-α treatment in individuals with rheumatoid arthritis: A pilot study. Scand. J. Rheumatol. 2013, 42, 356–361.
- Oda, R.; Fujiwara, H.; Tokunaga, D.; Nakamura, S.; Taniguchi, D.; Kawahito, Y.; Seno, T.; Matsui, T.; Kubo, T. How do anti-TNF therapies affect gait function in patients with rheumatoid arthritis? Int. J. Rheum. Dis. 2014, 17, 57–62.
- McDermott, M.M.; Liu, K.; Ferrucci, L.; Tian, L.; Guralnik, J.M.; Green, D.; Tan, J.; Liao, Y.; Pearce, W.H.; Schneider, J.R.; et al. Circulating Blood Markers and Functional Impairment in Peripheral Arterial Disease. J. Am. Geriatr. Soc. 2008, 56, 1504–1510.
- Brinkley, T.E.; Leng, X.; Miller, M.E.; Kitzman, D.W.; Pahor, M.; Berry, M.J.; Marsh, A.P.; Kritchevsky, S.B.; Nicklas, B.J. Chronic Inflammation Is Associated with Low Physical Function in Older Adults Across Multiple Comorbidities. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 455–461.
- Hsu, F.-C.; Kritchevsky, S.B.; Liu, Y.; Kanaya, A.; Newman, A.B.; Perry, S.E.; Visser, M.; Pahor, M.; Harris, T.B.; Nicklas, B.J.; et al. Association Between Inflammatory Components and Physical Function in the Health, Aging, and Body Composition Study: A Principal Component Analysis Approach. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64A, 581–589.
- Penninx, B.W.J.H.; Kritchevsky, S.B.; Newman, A.B.; Nicklas, B.J.; Simonsick, E.M.; Rubin, S.; Nevitt, M.; Visser, M.; Harris, T.; Pahor, M. Inflammatory Markers and Incident Mobility Limitation in the Elderly. J. Am. Geriatr. Soc. 2004, 52, 1105–1113.
- Ferrucci, L.; Harris, T.B.; Guralnik, J.M.; Tracy, R.P.; Corti, M.-C.; Cohen, H.J.; Penninx, B.; Pahor, M.; Wallace, R.; Havlik, R.J. Serum IL-6 Level and the Development of Disability in Older Persons. J. Am. Geriatr. Soc. 1999, 47, 639–646.
- Chung, H.Y.; Cesari, M.; Anton, S.; Marzetti, E.; Giovannini, S.; Seo, A.Y.; Carter, C.; Yu, B.P.; Leeuwenburgh, C. Molecular inflammation: Underpinnings of aging and age-related diseases. Ageing Res. Rev. 2009, 8, 18–30.
- Franceschi, C.; Campisi, J. Chronic Inflammation (Inflammaging) and Its Potential Contribution to Age-Associated Diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9.
- Ravaglia, G.; Forti, P.; Maioli, F.; Brunetti, N.; Martelli, M.; Talerico, T.; Bastagli, L.; Muscari, A.; Mariani, E. Peripheral blood markers of inflammation and functional impairment in elderly community-dwellers. Exp. Gerontol. 2004, 39, 1415–1422.
- Michaud, M.; Balardy, L.; Moulis, G.; Gaudin, C.; Peyrot, C.; Vellas, B.; Cesari, M.; Nourhashemi, F. Proinflammatory Cytokines, Aging, and Age-Related Diseases. J. Am. Med. Dir. Assoc. 2013, 14, 877–882.
- Verghese, J.; Holtzer, R.; Lipton, R.B.; Wang, C. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 2012, 41, 541–545.
- Kuo, H.-K.; Bean, J.F.; Yen, C.-J.; Leveille, S.G. Linking C-Reactive Protein to Late-Life Disability in the National Health and Nutrition Examination Survey (NHANES) 1999–2002. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 380–387.
- Liu, J.; Yin, W.; Zhou, C.; Zhu, Y.; Gu, M.; Liu, B.; Ren, H.; Yang, X. Association between levels of high-sensitivity C-reactive protein in plasma and freezing of gait in Parkinson’s disease. Aging Clin. Exp. Res. 2022, 34, 1865–1872.
- Dupont, J.; Antonio, L.; Dedeyne, L.; O’Neill, T.W.; Vanderschueren, D.; Rastrelli, G.; Maggi, M.; Bártfai, G.; Casanueva, F.F.; Giwercman, A.; et al. Inflammatory markers are associated with quality of life, physical activity, and gait speed but not sarcopenia in aged men (40–79 years). J. Cachexia Sarcopenia Muscle 2021, 12, 1818–1831.
- Langmann, G.A.; Perera, S.; Ferchak, M.A.; Nace, D.A.; Resnick, N.M.; Greenspan, S.L. Inflammatory Markers and Frailty in Long-Term Care Residents. J. Am. Geriatr. Soc. 2017, 65, 1777–1783.
- Lassale, C.; Batty, G.D.; Steptoe, A.; Cadar, D.; Akbaraly, T.N.; Kivimäki, M.; Zaninotto, P. Association of 10-Year C-Reactive Protein Trajectories with Markers of Healthy Aging: Findings from the English Longitudinal Study of Aging. J. Gerontol. Ser. A 2019, 74, 195–203.
- Kositsawat, J.; Barry, L.C.; Kuchel, G.A. C-Reactive Protein, Vitamin D Deficiency, and Slow Gait Speed. J. Am. Geriatr. Soc. 2013, 61, 1574–1579.
- Beavers, D.P.; Kritchevsky, S.B.; Gill, T.M.; Ambrosius, W.T.; Anton, S.D.; Fielding, R.A.; King, A.C.; Rejeski, W.J.; Lovato, L.; McDermott, M.M.; et al. Elevated IL-6 and CRP Levels Are Associated with Incident Self-Reported Major Mobility Disability: A Pooled Analysis of Older Adults with Slow Gait Speed. J. Gerontol. Ser. A 2021, 76, 2293–2299.
- Heumann, Z.; Youssim, I.; Kizony, R.; Friedlander, Y.; Shochat, T.; Weiss, R.; Hochner, H.; Agmon, M. The Relationships of Fibrinogen and C-Reactive Protein with Gait Performance: A 20-Year Longitudinal Study. Front. Aging Neurosci. 2022, 14, 761948.
- Bai, A.; Shi, H.; Huang, X.; Xu, W.; Deng, Y. Association of C-Reactive Protein and Motoric Cognitive Risk Syndrome in Community-Dwelling Older Adults: The China Health and Retirement Longitudinal Study. J. Nutr. Health Aging 2021, 25, 1090–1095.
- Dupont, J.; Vercauteren, L.; Amini, N.; Lapauw, L.; De Schaepdryver, M.; Poesen, K.; Dedeyne, L.; Verschueren, S.; Tournoy, J.; Koppo, K.; et al. Are inflammatory markers associated with sarcopenia-related traits in older adults with sarcopenia?—A cross-sectional analysis of the ENHANce study. Exp. Gerontol. 2023, 178, 112196.
- Brown, P.J.; Roose, S.P.; Zhang, J.; Wall, M.; Rutherford, B.R.; Ayonayon, H.N.; Butters, M.A.; Harris, T.; Newman, A.B.; Satterfield, S.; et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 221–227.
- Kim, M.; Won, C.W. Sarcopenia Is Associated with Cognitive Impairment Mainly Due to Slow Gait Speed: Results from the Korean Frailty and Aging Cohort Study (KFACS). Int. J. Environ. Res. Public Health 2019, 16, 1491.
- Custodero, C.; Pahor, M.; Mazzoccoli, C.; Manini, T.M.; Anton, S.D.; Mazzocca, A.; Lozupone, M.; Panza, F.; Sabbà, C.; Solfrizzi, V. Effect of change of interleukin-6 over time on gait speed response: Results from the lifestyle interventions and independence for elders study. Mech. Ageing Dev. 2023, 210, 111763.
- Felicio, D.C.; Pereira, D.S.; Assumpção, A.M.; de Jesus-Moraleida, F.R.; de Queiroz, B.Z.; da Silva, J.P.; Rosa, N.M.d.B.; Dias, J.M.D.; Pereira, L.S.M. Inflammatory mediators, muscle and functional performance of community-dwelling elderly women. Arch. Gerontol. Geriatr. 2014, 59, 549–553.
- Mezuk, B.; Lohman, M.; Dumenci, L.; Lapane, K.L. Are Depression and Frailty Overlapping Syndromes in Mid- and Late-life? A Latent Variable Analysis. Am. J. Geriatr. Psychiatry 2013, 21, 560–569.
- Nadkarni, N.K.; Boudreau, R.M.; Studenski, S.A.; Lopez, O.L.; Liu, G.; Kritchevsky, S.; Yaffe, K.; Newman, A.B.; Rosano, C. Slow gait, white matter characteristics, and prior 10-year interleukin-6 levels in older adults. Neurology 2016, 87, 1993–1999.
- Sipilä, S.; Tirkkonen, A.; Savikangas, T.; Hänninen, T.; Laukkanen, P.; Alen, M.; Fielding, R.A.; Kivipelto, M.; Kulmala, J.; Rantanen, T.; et al. Effects of physical and cognitive training on gait speed and cognition in older adults: A randomized controlled trial. Scand. J. Med. Sci. Sports 2021, 31, 1518–1533.
- Rasmussen, L.J.H.; Caspi, A.; Ambler, A.; Broadbent, J.M.; Cohen, H.J.; d’Arbeloff, T.; Elliott, M.; Hancox, R.J.; Harrington, H.; Hogan, S.; et al. Association of Neurocognitive and Physical Function with Gait Speed in Midlife. JAMA Netw. Open 2019, 2, e1913123.
- Kyrdalen, I.L.; Thingstad, P.; Sandvik, L.; Ormstad, H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother. Res. Int. 2019, 24, e1743.
- Sui, S.X.; Holloway-Kew, K.L.; Hyde, N.K.; Williams, L.J.; Leach, S.; Pasco, J.A. Muscle strength and gait speed rather than lean mass are better indicators for poor cognitive function in older men. Sci. Rep. 2020, 10, 10367.
- Fornage, M.; Chiang, Y.A.; O’Meara, E.S.; Psaty, B.M.; Reiner, A.P.; Siscovick, D.S.; Tracy, R.P.; Longstreth, W.T., Jr. Biomarkers of Inflammation and MRI-Defined Small Vessel Disease of the Brain. Stroke 2008, 39, 1952–1959.
- Satizabal, C.L.; Zhu, Y.C.; Mazoyer, B.; Dufouil, C.; Tzourio, C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: The 3C-Dijon Study. Neurology 2012, 78, 720–727.
- Wersching, H.; Duning, T.; Lohmann, H.; Mohammadi, S.; Stehling, C.; Fobker, M.; Conty, M.; Minnerup, J.; Ringelstein, E.; Berger, K.; et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology 2010, 74, 1022–1029.
- Benson, R.R.; Guttmann, C.R.G.; Wei, X.; Warfield, S.K.; Hall, C.; Schmidt, J.A.; Kikinis, R.; Wolfson, L.I. Older people with impaired mobility have specific loci of periventricular abnormality on MRI. Neurology 2002, 58, 48–55.
- Rosano, C.; Kuller, L.H.; Chung, H.; Arnold, A.M.; Longstreth, W.T.; Newman, A.B. Subclinical Brain Magnetic Resonance Imaging Abnormalities Predict Physical Functional Decline in High-Functioning Older Adults. J. Am. Geriatr. Soc. 2005, 53, 649–654.
- Tan, L.F.; Chan, Y.H.; Seetharaman, S.; Denishkrshna, A.; Au, L.; Kwek, S.C.; Chen, M.Z.; Ng, S.E.; Hui, R.J.Y.; Merchant, R.A. Impact of Exercise and Cognitive Stimulation Therapy on Physical Function, Cognition and Muscle Mass in Pre-Frail Older Adults in the Primary Care Setting: A Cluster Randomized Controlled Trial. J. Nutr. Health Aging 2023, 27, 438–447.
- Renner, S.W.; Qiao, Y.; Gmelin, T.; Santanasto, A.J.; Boudreau, R.M.; Walston, J.D.; Perls, T.T.; Christensen, K.; Newman, A.B.; Glynn, N.W.; et al. Association of fatigue, inflammation, and physical activity on gait speed: The Long Life Family Study. Aging Clin. Exp. Res. 2022, 34, 367–374.
- Scalzo, P.; Kümmer, A.; Cardoso, F.; Teixeira, A.L. Serum levels of interleukin-6 are elevated in patients with Parkinson’s disease and correlate with physical performance. Neurosci. Lett. 2010, 468, 56–58.
More
Information
Subjects:
Biology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
797
Revisions:
2 times
(View History)
Update Date:
23 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




