Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Soraya Ratnawulan Mita | -- | 2486 | 2024-02-20 15:59:50 | | | |
| 2 | Camila Xu | Meta information modification | 2486 | 2024-02-22 06:58:18 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Mita, S.R.; Husni, P.; Putriana, N.A.; Maharani, R.; Hendrawan, R.P.; Dewi, D.A. Physicochemical Properties and Activities of Catechins. Encyclopedia. Available online: https://encyclopedia.pub/entry/55242 (accessed on 08 March 2026).
Mita SR, Husni P, Putriana NA, Maharani R, Hendrawan RP, Dewi DA. Physicochemical Properties and Activities of Catechins. Encyclopedia. Available at: https://encyclopedia.pub/entry/55242. Accessed March 08, 2026.
Mita, Soraya Ratnawulan, Patihul Husni, Norisca Aliza Putriana, Rani Maharani, Ryan Proxy Hendrawan, Dian Anggraeni Dewi. "Physicochemical Properties and Activities of Catechins" Encyclopedia, https://encyclopedia.pub/entry/55242 (accessed March 08, 2026).
Mita, S.R., Husni, P., Putriana, N.A., Maharani, R., Hendrawan, R.P., & Dewi, D.A. (2024, February 20). Physicochemical Properties and Activities of Catechins. In Encyclopedia. https://encyclopedia.pub/entry/55242
Mita, Soraya Ratnawulan, et al. "Physicochemical Properties and Activities of Catechins." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Catechins are bioactive polyphenols and are typically isolated from green tea (Camellia sinensis L.) and gambir leaves (Uncaria gambir Roxb). There are a few types of catechins which possess the flavan-3-ol structure consisting of two benzene rings, a heterocycle dihydropyran, and a hydroxyl.
catechins
flavonoid
antioxidant
cosmeceutical
1. Introduction
Cosmetics are widely used by everyone and can be classified as decorative or skincare cosmetics. They are defined by BPOM Indonesia as materials or preparations intended for use on the external parts of the human body such as the epidermis, hair, nails, lips, and external genital organs, or on the teeth and oral mucous membranes, especially for cleaning, perfuming, changing the appearance and/or improving body odour, or to protect or maintain the body in good condition. Based on the literature, cosmetics are often used for aesthetic and self-care benefits, and that they also intersect with skin health. The skin is the largest human organ and protects the body from external insults, therefore, it is essential to care for the skin according to the unique needs of the individual. The term ‘cosmeceutical’ refers to a cosmetic product that provides medical effects and contains certain active compounds. These cosmeceutical products are not only for whitening, antiageing, and sunscreen purposes, but also for hyperpigmentation, photoageing, wrinkles, and hair loss [1]. It is important to note that cosmeceutical preparations are not intended to be given systematically, rather, they are applied locally/topically, that is, the ‘dermal delivery’ as the site of action is usually the stratum corneum (SC), the viable epidermis and/or dermis [2].
Skin ageing typically starts to manifest when an individual reaches their late 20s to early 30s due to two distinct sources [3]: intrinsic ageing, which results from issues within the network of elastin fibres and collagen, or extrinsic ageing due to exposure to environmental factors such as sun radiation. Oxidative stress triggers inflammation, constraining epidermal cell renewal and ultimately leads to a reduction in epidermal thickness and a weakening of the protective barrier [4]. Sun radiation triggers the creation of reactive oxygen radicals (ROS) that cause keratinocytes to generate pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-8 (IL-8), thereby producing more ROS [5]. Excessive ROS leads to the development of wrinkles by causing the breakdown and abnormal interlinking of structural proteins such as glycosaminoglycans, collagen, and elastin fibres in the skin’s extracellular matrix. Hence, antioxidants isolated from natural products can be used to suppress ROS production to slow down skin ageing [6].
Catechins are natural flavan-3-ols (or flavonols), a type of polyphenolic compound belonging to the flavonoid family. They are present in a variety of fruits, vegetables, and plant-based beverages [7] and are particularly concentrated in tea leaves, red wine, broad beans, rock-rose leaves, apricots, black grapes, and strawberries. Epicatechin is abundant in chocolate, apples, broad beans, pears, black grapes, cherries, and certain types of berries including blackberries and rasp6berries [8].
Catechins offer numerous health benefits by effectively eliminating free radicals and slowing down the breakdown of the extracellular matrix caused by exposure to ultraviolet (UV) radiation and pollution. They stimulate collagen production while preventing the generation of matrix metalloproteinase enzymes. Due to the presence of hydroxyl in the gallate group, epigallocatechin gallate (EGCG) and epigallocatechin (ECG) can neutralise free radicals, surpassing several antioxidants like trolox, ascorbic acid, and tocopherol [9]. Thus, catechins have the potential to be used in cosmetic and dermatological products [10] and are now commonly included in pharmaceutical, medical, and cosmetic products. For example, a transethosomal gel form of catechins can reduce total cholesterol in mice [11] and Uncaria gambir is used to treat diarrhoea, sore throat, spongy gums, dysentery, arteriosclerosis, and obesity [12].
2. Physicochemical Properties of Catechins
Catechins are bioactive polyphenols and are typically isolated from green tea (Camellia sinensis L.) and gambir leaves (Uncaria gambir Roxb). There are a few types of catechins which possess the flavan-3-ol structure consisting of two benzene rings, a heterocycle dihydropyran, and a hydroxyl [13][14]. The structures are shown in Figure 1.
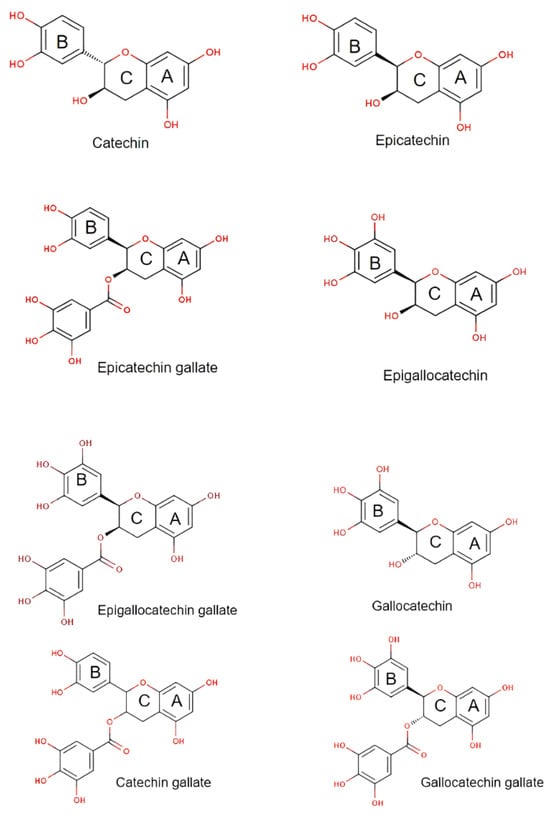
Figure 1. Various type of catechin structures.
Catechins have been utilised in many pharmacological formulations [15][16]. The use of catechins is based on its physicochemical characteristics such as polarisability, dipole moment, molecular weight, surface area, and van der Waals volume, as well as macroscopic traits including solubility, octanol/water partition coefficient, acidity or basicity in solution, etc. [17]. In the Indonesian standard guide, namely the Indonesian Herbal Pharmacopoeia, there are several physicochemical profiles of catechins obtained from gambir plants (Uncaria gambir (Hunter) Roxb.) as shown in Table 1.
Table 1. Physicochemical parameters of catechins based on the Pharmacopeia handbook.
| Physicochemical Parameters |
Accepted Value | Reference |
|---|---|---|
| Organoleptic | Solid form, it appears as a light brown to dark reddish-brown substance with a distinctive odour. It possesses a chelate taste that is slightly bitter at first but ends with a sweet aftertaste. | [18] |
| Water Content | Quantity should not exceed 14% | [18] |
| Ash Content | Quantity should not exceed 0.5% | [18] |
| Ash, not soluble in acid | Quantity should not exceed 0.1% | [18] |
| Purity | Contains no less than 90% tannins counted as catechins. | [18] |
| Identification | The assay was carried out using spectrophotometry with a wavelength of 294 nm. | [18] |
| Molecular weight | 290.27 g/mol | [18] |
| Solubility | Soluble in water and polar organic solvents; soluble in pressurised hot water between 298.75 to 415.85 K; soluble in mixtures of supercritical carbon dioxide (SC-CO2) and ethanol at 313 K and pressures ranging from 80 to 120 bar; soluble in SC-CO2 between 313.15 and 343.15 K and pressures ranging from 12 to 26 MPa using ethanol as the co-solvent. | [19] |
3. Activities of Catechin
3.1. Antioxidant
Antioxidants scavenge oxygen free radicals (singlet and triplet), ROS, peroxide decomposers, and enzyme inhibitors to protect vital molecules from harm [20]. The use of antioxidants derived from natural ingredients is increasing. Natural antioxidants can be single pure compounds/isolates, combinations of compounds, or plant extracts. The secondary metabolites, polyphenols, are the most common phyto-antioxidants [21]. Polyphenols have benzene rings with attached -OH groups which determine the antioxidant activity based on their number and position [22]. Protein phosphorylation is influenced by phenol groups by inhibiting lipid peroxidation. Flavonoids are the main source of polyphenols, while carotenoids are the most abundant sources of terpenes [23].
Catechin produces and discards free radicals [24] through several key direct and indirect antioxidant mechanisms. The direct mechanism involves the scavenging of ROS, whereas the indirect mechanism occurs through increased antioxidant enzymes and the inhibition of the pro-enzyme that participates in oxidant stress [8][25]. The phenolic hydroxyl group in catechin is involved in the scavenging of ROS, therefore more hydroxyl groups will improve the antioxidant activity. According to the structure, the hierarchy of antioxidant activity of catechins is EGCG, EGG, EGC, EC, and, lastly, catechin [8].
The structural characteristics of flavan-3-ols, specifically their resorcinol and catechol components, which consist of A and B rings connected by the Pyron ring (C ring), are responsible for their antioxidant properties (Figure 1) [26]. The ability of flavan-3-ols to scavenge radicals primarily relies on the arrangement of hydroxyl groups and their capacity to donate hydrogen atoms [27]. The stability of the phenoxy radical produced after hydrogen atom transfer (HAT) also plays a role in their ability to counteract reactive oxygen radicals [28]. Catechins can exist in four different diastereoisomers, which arise from two chiral centers (2n) at C2 and C3. These diastereoisomers are referred to as (+) catechin (2R, 3S), (−) epicatechin (2R, 3R), (−) catechin (2S, 3R), and (+) epicatechin (2S, 3S) [29]. Overall, the stereoisomerisms are determined by the positioning of the B ring connected to the C ring at the C2 atom and also the chirality of R1 and R2 attached to the C ring at the C3.
The structures are indicative of a site of a projected bond—R stands for dashed wedge bonds that extend away from the viewer, and S represents solid wedge bonds that project out of the paper towards the viewer, as seen in Figure 2. The degree of polymerization also plays a role in determining the antioxidant properties [30]. The capability of catechin and epicatechin to scavenge radicals is attributed to the dihydroxyl group at C-3’ and C-4’ on the B ring, a C3-hydroxyl group on the C ring without a 2,3 double bond, and hydroxyl groups at C5 and C7 on ring A. In this context, the catechol ring (B) exhibits greater electron-donating capacity than the other rings because it contains an ortho-dihydroxyl group (detail see Figure 2 and Figure 3) [31]. The scavenging of free radicals by the hydroxyl groups occurs via hydrogen atom transfer and single-electron atom transfer, with catechin undergoing oxidation to form the relatively reactive quinone [32] as could be observed from Figure 4.
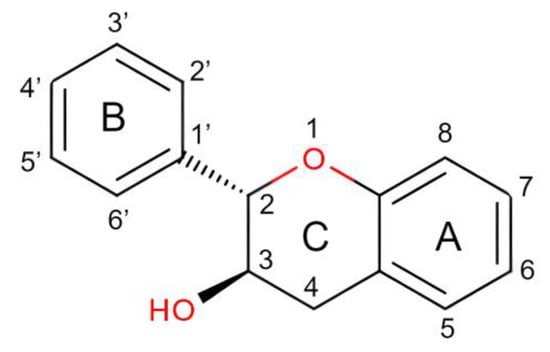
Figure 2. Structure of Catechin in Detail.
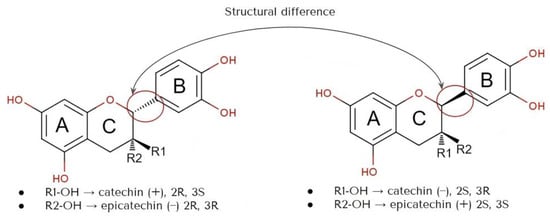
Figure 3. Structure of stereoisomers of catechin (+) and epicatechin (−), also catechin (−) and epicatechin (+).
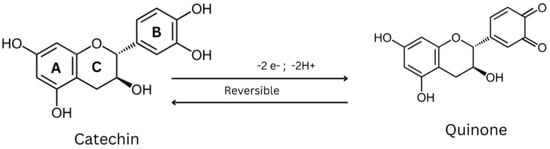
Figure 4. Scavenging of free radicals by catechin.
Numerous studies have reported that the primary source of antioxidant activity in CT and ECT is their elevated redox characteristics. The antioxidant properties of catechin and epicatechin, which are found in different plant extracts, were assessed using various experimental antioxidant tests, including ferric reducing antioxidant power (FRAP), 1,1-diphenyl-1-picrylhydrazyl (DPPH), nitric oxide (NO), and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS+), demonstrating that CT and ECT exhibit strong antioxidant capabilities [33]. CT and ECT share a similar molecular structure, but they exhibit distinct chemical reactivity properties because CT’s antioxidant capability relies on its planar geometry, whereas ECT’s antioxidant activity is due to the interaction of hydrogen bonds at the catechol moiety. The lack of a double bond at C2 = C3 in the C ring significantly affects ECT’s antioxidant capacity. Additionally, it has been demonstrated that the reactivity of flavan-3-ols is thermodynamically modified depending on the solvents used [34].
There are many uses of catechins. Aside from being used as pure antioxidant compounds, catechins can be formulated into many products including sunscreen, lip balm, anti-dandruff shampoo, cosmetic cleansers, and cosmetic creams. They have also been extracted from many natural sources, as detailed in Table 2.
Table 2. Antioxidant activity of catechins from various sources.
| Methods | Sample | Result | Ref. |
|---|---|---|---|
| DPPH Assay | Camellia sinensis |
|
[35] |
| Lepisanthes alata (Blume) Leenh |
|
[36] | |
|
|||
|
|||
| Sterculia quadrifida R. |
Bark: 51.5 µg/mL (50%)
|
[20] | |
| Malondialdehyde | Uncaria Gambir Roxb |
|
[37] |
| Uncaria Gambir Roxb. |
Varies from 2.732% to 3.792%
|
[38] | |
| Combination of Isolated Catechin and Quercetin |
|
[39] |
In the context of the skin, several studies indicate that antioxidants play a crucial role in inhibiting the effects of radiation [40]. The various roles of antioxidants on the skin are presented in Table 3.
Table 3. The various roles of antioxidants on skin.
| Antioxidant Function | Mechanism of Action | Reference |
|---|---|---|
| Anti-ageing | Antioxidants inhibit the action of superoxide dismutase (SOD) enzymes, which play a role in degrading collagen. | [41] |
| Skin Brightening | Antioxidants have whitening effects by inhibiting tyrosinase and act as anti-inflammatory agents for hyperpigmentation caused by UV exposure (commonly known as melasma). | [42] |
| UV Filters | Topically administered antioxidants can enhance the photoprotective capabilities of UV filters by reducing erythema, inhibiting the development of sunburned skin cells, and causing immunosuppression. | [43] |
| Skin Hydration and Anti-Hyperpigmentation | Antioxidants suppress the production and secretion of melanin in melanoma cells to enhance skin hydration and improve hyperpigmentation. | [44] |
3.2. Anti-Ageing
Elastase, a protein kinase enzyme, cleaves specific polypeptide bonds to reduce elastin levels. Preventing elastase functions within the dermis layer can be utilised to maintain the skin’s flexibility, therefore, elastase activity inhibitors can serve as cosmetic ingredients to counteract the signs of skin ageing [45]. Polyphenols found in isolated white tea inhibit collagenase and elastase enzymes, specifically catechin and EGCG (Figure 5). Furthermore, given that collagenase is a zinc-containing metalloproteinase, these catechins could potentially attach to the Zn2+ ion present in the enzyme, thereby obstructing its ability to bind to the substrate [46].
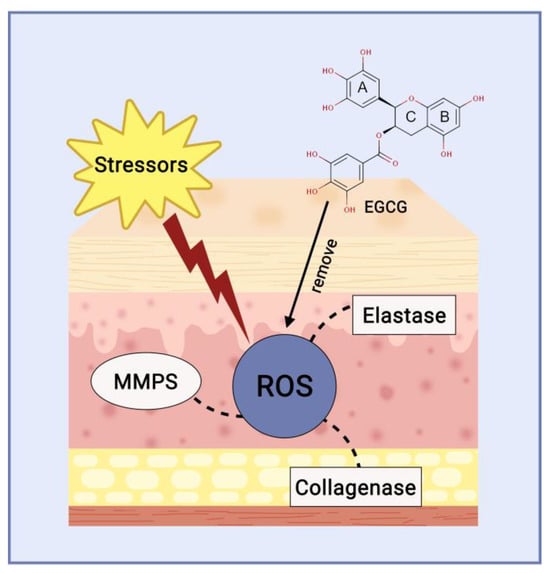
Figure 5. Catechin mechanism of action as anti-ageing agent.
3.3. Skin Brightener
Catechin directly inhibits tyrosinase activity and reduces the expression of tyrosinase [47]. EGCG, GCG, and EGC demonstrate great potential as tyrosinase activity inhibitors [48]. The catechins have a substantial inhibitory effect on tyrosinase activity and melanin production by downregulating the cAMP/CREB/MITF signalling pathway in B16F10 cells (Figure 6), with ECG demonstrating the strongest effect, followed by EGCG and GCG [49]. EGCG also suppresses the production of melanin induced by α-MSH in B16 melanoma cells [47].
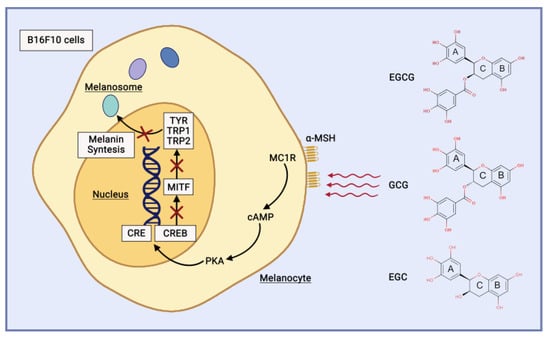
Figure 6. Catechin mechanism of action as anti-hyperpigmentation and brightening agent.
3.4. Anti-Hyperpigmentation
UV radiation results in melanogenesis. The mass production of melanin in the skin minimises UV radiation but causes the skin to slightly darken to a brownish color. Catechin as a depigmentation agent will inhibit melanin formation by inhibiting melanin synthesis through tyrosinase (TYR) and microphthalmia-associated transcription factor (MITF). MITF is a transcription factor that is responsible for melanocyte development in melanogenesis. Catechin will inhibit MILTF, thereby preventing melanocytes from producing melanin and hyperpigmentation [50].
3.5. UV-Reduction and Sunscreen
UV radiation is categorised into UV-A (315–400 nm), UV-B (280–315 nm), and UV-C (280–100 nm). Continuous exposure to UV-B radiation can lead to disruptions in the skin caused by free radicals and ROS, which stimulate melanin production and melanocyte proliferation. Tyrosinase facilitates melanin synthesis and represents a pivotal point in melanogenesis. High melanin levels can disrupt pigmentation in human skin, leading to conditions such as age spots, melasma, malignant melanomas, and freckles. EGCG is acknowledged as a natural antioxidant to neutralise free radicals, modulate the activity of antioxidant enzymes, diminish the effects of oxidative stress, and inhibit tyrosinase activity [51].
Sunscreen is a cosmetic that functions as a skin protector [52], filtering UV to reduce the radiation emitted from the sun [53][54]. Aromatic compounds conjugated to carbonyl groups convert UV energy into minimised UV energy [49], preventing the chemical properties of UV-absorbing potency without the need for significant photodegradation (Figure 7). The usage of sunscreen is determined based on the Sun Protection Factor (SPF), which is defined as the ability to supply a minimal erythema dose (MED) on the skin divided by the variable of UV energy that is needed to supply MED on unprotected skin [55].
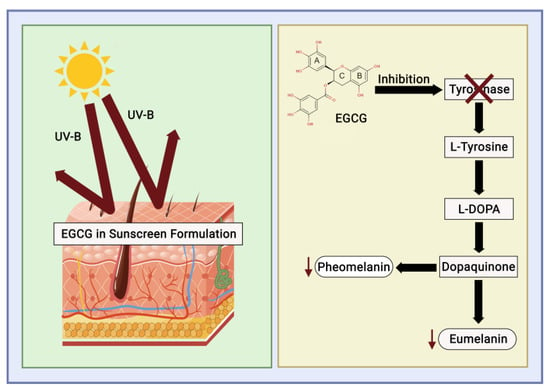
Figure 7. Catechin mechanism of action as a sunscreen.
The higher the SPF, the more protective the sunscreen [56] to prevent sunburn as well as skin cancer in the long term. The SPF can be determined with in vivo or in vitro methods. The in vivo test involves human volunteers while the in vitro is differentiated through two categories: measuring the UV absorption or transmission of the test product or spectrophotometric analysis. Effective sunscreen products at least need to have an absorbance of 290 to 400 mm [57].
3.6. Anti-Acne
Acne is a skin disease caused by the overgrowth of Staphylococcus epidermidis and Propionibacterium acnes. Some recent research showed that catechin from gambir and green tea could inhibit the growth of acne pathogens. Recent research showed the MIC of catechin as anti-acne is 0.1%. Another study showed that the inhibition zone of catechin is 18.45 mm for P. acnes and 15.68 mm for S. epidermidis [58]. The antibacterial mechanism of catechin involves disrupting the cell membrane and inhibiting intracellular enzymes (Figure 8). The mechanism of catechin to counter acne inflammation is through the inhibition of the pro-inflammatory cytokines IL-8 and TNF-α [59].
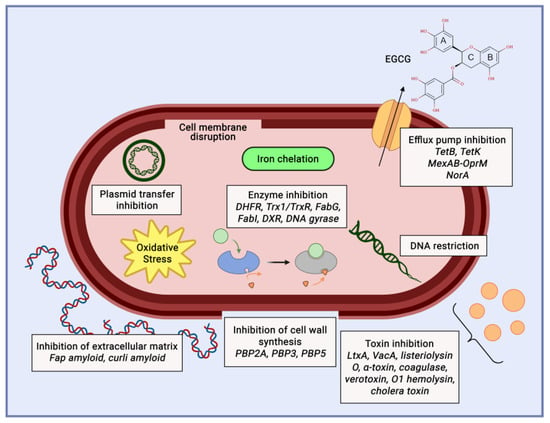
Figure 8. Catechin mechanism of action as anti-acne agent.
References
- Bellad, K.A.; Nanjwade, B.K.; Kamble, M.S.; Srichana, T.; Idris, N.F. Development of cosmeceuticals. World J. Pharm. Pharm. Sci. 2017, 6, 643–691.
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and controversies in studying sunscreen delivery and toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86.
- Cavinato, M.; Jansen-Durr, P. Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin. Exp. Gerontol. 2017, 94, 78–82.
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative stress in aging human skin. Biomolecules 2015, 5, 545–589.
- Chang, H.H.; Chien, C.Y.; Chen, K.H.; Huang, S.C.; Chien, C.T. Catechins Blunt the Effects of oxLDL and its Primary Metabolite Phosphatidylcholine Hydroperoxide on Endothelial Dysfunction Through Inhibition of Oxidative Stress and Restoration of eNOS in Rats. Kidney Blood Press. Res. 2017, 42, 919–932.
- Lee, S.; Yu, J.S.; Phung, H.M.; Lee, J.G.; Kim, K.H.; Kang, K.S. Potential Anti-Skin Aging Effect of (-)-Catechin Isolated from the Root Bark of Ulmus davidiana var. japonica in Tumor Necrosis Factor-alpha-Stimulated Normal Human Dermal Fibroblasts. Antioxidants 2020, 9, 981.
- Braicu, C.; Ladomery, M.R.; Chedea, V.S.; Irimie, A.; Berindan-Neagoe, I. The relationship between the structure and biological actions of green tea catechins. Food Chem. 2013, 141, 3282–3289.
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965.
- Matsubara, T.; Wataoka, I.; Urakawa, H.; Yasunaga, H. Effect of reaction pH and CuSO4 addition on the formation of catechinone due to oxidation of (+)-catechin. Int. J. Cosmet. Sci. 2013, 35, 362–367.
- Ferreira-Nunes, R.; da Silva, S.M.M.; de Souza, P.E.N.; de Oliveira Magalhães, P.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Incorporation of Eugenia dysenterica extract in microemulsions preserves stability, antioxidant effect and provides enhanced cutaneous permeation. J. Mol. Liq. 2018, 265, 408–415.
- Mita, S.R.; Abdassah, M.; Supratman, U.; Shiono, Y.; Rahayu, D.; Sopyan, I.; Wilar, G. Nanoparticulate System for the Transdermal Delivery of Catechin as an Antihypercholesterol: In Vitro and In Vivo Evaluations. Pharmaceuticals 2022, 15, 1142.
- Munggari, I.P.; Kurnia, D.; Deawati, Y.; Julaeha, E. Current Research of Phytochemical, Medicinal and Non-Medicinal Uses of Uncaria gambir Roxb.: A Review. Molecules 2022, 27, 6551.
- Bae, J.; Kim, N.; Shin, Y.; Kim, S.-Y.; Kim, Y.-J. Activity of catechins and their applications. Biomed. Dermatol. 2020, 4, 1–10.
- Isemura, M. Catechin in Human Health and Disease. Molecules 2019, 24, 528.
- Syukri, D.; Azima, F.; Aprialdho, R. Study on the Utilization of Catechins from Gambir (Uncaria Gambir Roxb) Leaves as Antioxidants Cooking Oil. Andalasian Int. J. Agric. Nat. Sci. 2022, 3, 12–25.
- Zillich, O.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464.
- Moldoveanu, S.C.; David, V. Essentials in Modern HPLC Separations; Elsevier: Amsterdam, The Netherlands, 2022.
- Hariyati, N. Ministry of Health of the Republic of Indonesia Farmakope Herbal Indonesia, 2nd ed.; Ministry of Health Indonesia: Kota Jakarta, Indonesia, 2017.
- Cuevas-Valenzuela, J.; González-Rojas, Á.; Wisniak, J.; Apelblat, A.; Pérez-Correa, J.R. Solubility of (+)-catechin in water and water-ethanol mixtures within the temperature range 277.6–331.2 K: Fundamental data to design polyphenol extraction processes. Fluid Phase Equilibria 2014, 382, 279–285.
- Riwu, A.G.; Nugraha, J.; Purwanto, D.A.; Triyono, E.A. Determination of (+)-Catechin and Antioxidant Activity in Faloak (Sterculia quadrifida R. Br) Stem Bark Infusion. Sci. Technol. Indones. 2023, 8, 59–65.
- Delarosa, A.; Hendrawan, R.P.; Halimah, E. Screening of Costus speciosus and Determination of Antioxidant Potential Using DPPH Method: A Review. Eur. J. Med. Plants 2023, 34, 17–28.
- Chen, Z.; Liu, Q.; Zhao, Z.; Bai, B.; Sun, Z.; Cai, L.; Fu, Y.; Ma, Y.; Wang, Q.; Xi, G. Effect of hydroxyl on antioxidant properties of 2, 3-dihydro-3, 5-dihydroxy-6-methyl-4 H-pyran-4-one to scavenge free radicals. RSC Adv. 2021, 11, 34456–34461.
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C.J.C. Natural antioxidants from plant extracts in skincare cosmetics: Recent applications, challenges and perspectives. Cosmetics 2021, 8, 106.
- Grzesik, M.; Naparło, K.; Bartosz, G.; Sadowska-Bartosz, I. Antioxidant properties of catechins: Comparison with other antioxidants. Food Chem. 2018, 241, 480–492.
- Sheng, Y.; Sun, Y.; Tang, Y.; Yu, Y.; Wang, J.; Zheng, F.; Li, Y.; Sun, Y. Catechins: Protective mechanism of antioxidant stress in atherosclerosis. Front. Pharmacol. 2023, 14, 1144878.
- Šeruga, M.; Tomac, I. Influence of chemical structure of some flavonols on their electrochemical behaviour. Int. J. Electrochem. Sci. 2017, 12, 7616–7637.
- Hassanpour, S.H.; Doroudi, A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J. Phytomed. 2023, 13, 354.
- Platzer, M.; Kiese, S.; Tybussek, T.; Herfellner, T.; Schneider, F.; Schweiggert-Weisz, U.; Eisner, P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front. Nutr. 2022, 9, 882458.
- Anitha, S.; Krishnan, S.; Senthilkumar, K.; Sasirekha, V. Theoretical investigation on the structure and antioxidant activity of (+) catechin and (−) epicatechin–a comparative study. Mol. Phys. 2020, 118, e1745917.
- Zhou, H.-C.; Tam, N.F.-y.; Lin, Y.-M.; Ding, Z.-H.; Chai, W.-M.; Wei, S.-D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606.
- Spiegel, M.; Andruniów, T.; Sroka, Z. Flavones’ and Flavonols’ Antiradical Structure–Activity Relationship—A Quantum Chemical Study. Antioxidants 2020, 9, 461.
- Munteanu, I.G.; Apetrei, C. Assessment of the Antioxidant Activity of Catechin in Nutraceuticals: Comparison between a Newly Developed Electrochemical Method and Spectrophotometric Methods. Int. J. Mol. Sci. 2022, 23, 8110.
- Doshi, P.; Adsule, P.; Banerjee, K.; Oulkar, D. Technology. Phenolic compounds, antioxidant activity and insulinotropic effect of extracts prepared from grape (Vitis vinifera L) byproducts. J. Food Sci. Technol. 2015, 52, 181–190.
- Dias, M.C.; Pinto, D.C.; Silva, A.M.S. Plant flavonoids: Chemical characteristics and biological activity. Molecules 2021, 26, 5377.
- Nuryana, I.; Ratnakomala, S.; Fahrurrozi, A.B.J.; Andriani, A.; Putra, F.J.N.; Rezamela, E.; Wulansari, R.; Prawira-Atmaja, M.I.; Lisdiyanti, P. Catechin Contents, Antioxidant and Antibacterial Activities of Different Types of Indonesian Tea (Camellia Sinensis). Ann. Bogor. 2020, 24, 107.
- Anggraini, T.; Wilma, S.; Syukri, D.; Azima, F.J.I.J.o.F.S. Total phenolic, anthocyanin, Catechins, DPPH radical scavenging activity, and toxicity of Lepisanthes alata (Blume) Leenh. Int. J. Food Sci. 2019, 2019, 9703176.
- Musdja, M.Y.; Rahman, H.A.; Hasan, D.J.L.I.J.H.L.-S. Antioxidant activity of catechins isolate of Uncaria gambier Roxb in male rats. LIFE Int. J. Health Life-Sci. 2018, 4, 34–46.
- Rahmi, M.; Rita, R.S.; Yetti, H. Gambir Catechins (Uncaria gambir Roxb) Prevent Oxidative Stress in Wistar Male Rats Fed a High-Fat Diet. Maj. Kedokt. Andalas 2021, 44, 436–441.
- Yetuk, G.; Pandir, D.; Bas, H. Protective role of catechin and quercetin in sodium benzoate-induced lipid peroxidation and the antioxidant system in human erythrocytes in vitro. Sci. World J. 2014, 2014, 874824.
- Amber, K.T.; Shiman, M.I.; Badiavas, E.V. The use of antioxidants in radiotherapy-induced skin toxicity. Integr. Cancer Ther. 2014, 13, 38–45.
- Zheng, M.; Liu, Y.; Zhang, G.; Yang, Z.; Xu, W.; Chen, Q. The Applications and Mechanisms of Superoxide Dismutase in Medicine, Food, and Cosmetics. Antioxidants 2023, 12, 1675.
- Boo, Y.C. Arbutin as a Skin Depigmenting Agent with Antimelanogenic and Antioxidant Properties. Antioxidants 2021, 10, 1129.
- Jesus, A.; Mota, S.; Torres, A.; Cruz, M.T.; Sousa, E.; Almeida, I.F.; Cidade, H. Antioxidants in Sunscreens: Which and What For? Antioxidants 2023, 12, 138.
- Nahhas, A.F.; Abdel-Malek, Z.A.; Kohli, I.; Braunberger, T.L.; Lim, H.W.; Hamzavi, I.H. The potential role of antioxidants in mitigating skin hyperpigmentation resulting from ultraviolet and visible light-induced oxidative stress. Photodermatol. Photoimmunol. Photomed. 2019, 35, 420–428.
- Andrade, J.M.; Domínguez-Martín, E.M.; Nicolai, M.; Faustino, C.; Rodrigues, L.M.; Rijo, P. Screening the dermatological potential of plectranthus species components: Antioxidant and inhibitory capacities over elastase, collagenase and tyrosinase. J. Enzyme Inhib. Med. Chem. 2021, 36, 258–270.
- Sonawane, G.B.; Jadhav, S.P.; Patil, C.D.; Kamble, P.R.; Somavanshi, D.B. A Review on the Antioxidant and Antiaging Properties of White Tea. J. Pharm. Res. Int. 2021, 33, 129–136.
- Wang, W.; Di, T.; Wang, W.; Jiang, H. EGCG, GCG, TFDG, or TSA inhibiting melanin synthesis by downregulating MC1R expression. Int. J. Mol. Sci. 2023, 24, 11017.
- Zhang, X.; Li, J.; Li, Y.; Liu, Z.; Lin, Y.; Huang, J.-A. Anti-melanogenic effects of epigallocatechin-3-gallate (EGCG), epicatechin-3-gallate (ECG) and gallocatechin-3-gallate (GCG) via down-regulation of cAMP/CREB/MITF signaling pathway in B16F10 melanoma cells. Fitoterapia 2020, 145, 104634.
- Jiang, T.; Qi, Y.; Wu, Y.; Zhang, J. Application of antioxidant and ultraviolet absorber into HDPE: Enhanced resistance to UV irradiation. e-Polymers 2019, 19, 499–510.
- Laksmiani, N.P.L.; Sanjaya, I.K.N.; Leliqia, N.P.E. The activity of avocado (Persea americana Mill.) seed extract containing catechin as a skin lightening agent. J. Pharm. Pharmacogn. Res. 2020, 8, 449–456.
- Vale, E.P.; dos Santos Morais, E.; de Souza Tavares, W.; de Sousa, F.F.O. Epigallocatechin-3-gallate loaded-zein nanoparticles: Characterization, stability and associated antioxidant, anti-tyrosinase and sun protection properties. J. Mol. Liq. 2022, 358, 119107.
- Donglikar, M.M.; Deore, S.L. Sunscreens: A review. Pharmacogn. J. 2016, 8, 171–179.
- Sander, M.; Sander, M.; Burbidge, T.; Beecker, J. The efficacy and safety of sunscreen use for the prevention of skin cancer. CMAJ 2020, 192, E1802–E1808.
- Dewi, D.A.R. Sunscreen Protection Against Visible Light: Is It Needed? Malahayati Nurs. J. 2022, 4, 2527–2536.
- Latha, M.; Martis, J.; Shobha, V.; Shinde, R.S.; Bangera, S.; Krishnankutty, B.; Bellary, S.; Varughese, S.; Rao, P.; Kumar, B.N.; et al. Sunscreening agents: A review. J. Clin. Aesthet. Dermatol. 2013, 6, 16.
- Portilho, L.; Aiello, L.M.; Vasques, L.I.; Bagatin, E.; Leonardi, G.R. Effectiveness of sunscreens and factors influencing sun protection: A review. Braz. J. Pharm. Sci. 2023, 58, e20693.
- Ebrahimzadeh, M.A.; Enayatifard, R.; Khalili, M.; Ghaffarloo, M.; Saeedi, M.; Charati, J.Y. Correlation between sun protection factor and antioxidant activity, phenol and flavonoid contents of some medicinal plants. Iran J. Pharm. Res. 2014, 13, 1041.
- Warnida, H.; Masliyana, A.; Sapri, S.J. Formulasi Ekstrak Etanol Gambir (Uncaria gambir Roxb.) dalam Bedak Anti Jerawat. J. Ilm. Manuntung 2016, 2, 99–106.
- Messire, G.; Serreau, R.; Berteina-Raboin, S. Antioxidant Effects of Catechins (EGCG), Andrographolide, and Curcuminoids Compounds for Skin Protection, Cosmetics, and Dermatological Uses: An Update. Antioxidants 2023, 12, 1317.
More
Information
Subjects:
Dermatology
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
1.8K
Revisions:
2 times
(View History)
Update Date:
22 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No





