Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | xuejia kang | -- | 2340 | 2024-02-20 01:31:42 | | | |
| 2 | Rita Xu | Meta information modification | 2340 | 2024-02-20 02:41:31 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Cheng, S.; Wang, H.; Kang, X.; Zhang, H. Immunotherapy Innovations in the Fight against Osteosarcoma. Encyclopedia. Available online: https://encyclopedia.pub/entry/55200 (accessed on 07 February 2026).
Cheng S, Wang H, Kang X, Zhang H. Immunotherapy Innovations in the Fight against Osteosarcoma. Encyclopedia. Available at: https://encyclopedia.pub/entry/55200. Accessed February 07, 2026.
Cheng, Shigao, Huiyuan Wang, Xuejia Kang, Hui Zhang. "Immunotherapy Innovations in the Fight against Osteosarcoma" Encyclopedia, https://encyclopedia.pub/entry/55200 (accessed February 07, 2026).
Cheng, S., Wang, H., Kang, X., & Zhang, H. (2024, February 20). Immunotherapy Innovations in the Fight against Osteosarcoma. In Encyclopedia. https://encyclopedia.pub/entry/55200
Cheng, Shigao, et al. "Immunotherapy Innovations in the Fight against Osteosarcoma." Encyclopedia. Web. 20 February, 2024.
Copy Citation
Immunosuppressive elements within the tumor microenvironment are the primary drivers of tumorigenesis and malignant advancement. The presence, as well as the crosstalk between myeloid-derived suppressor cells (MDSCs), osteosarcoma-associated macrophages (OS-Ms), regulatory T cells (Tregs), and endothelial cells (ECs) with osteosarcoma cells cause the poor prognosis of OS.
osteosarcoma
suppressive immune environment
nanoparticles
1. Introduction
Osteosarcoma, the most prevalent malignant bone-related cancer in adolescents, poses a significant treatment challenge [1]. The survival rate for this condition over a five-year period ranges from 60% to 70%, and it is more frequently observed in males and individuals of African American descent [2]. Despite extensive research efforts, 5-year survival rates for osteosarcoma patients have remained around 20% in recent decades [3]. What is worse, the prognosis for patients with metastatic or relapsed osteosarcoma has remained bleak and stagnant over the past 30 years [4]. The current management strategy for primary OS is surgery plus neoadjuvant chemotherapy (preoperative treatments), and the survival rate has been discovered as 35–40% [5][6]. Rotation-plasty, a well-established method for knee tumor reconstruction, can yield functional and psychological outcomes equal or superior to endoprosthetic reconstruction [7][8]. However, it presents cosmetic challenges [7][9]. Axial skeleton sarcoma surgery is complex due to high local recurrence risk and frequent reconstruction complications [7]. About 30% of primary metastatic osteosarcoma patients and over 40% achieving complete remission can be long-term survivors [10]. Lung metastases in advanced OS patients decrease the 5-year survival rate to 5–10%; in this case, the adjuvant combination of chemotherapy and immunotherapy significantly enhances survival rates. Despite this progress, the immunotherapy of OS still requires in-depth investigation.
The tumor microenvironment (TME) is a complex system composed of not only cancer cells, but also various cell types, including immune cells such as myeloid-derived suppressor cells (MDSCs), osteosarcoma-macrophage (OS-M), and stromal cells like the cancer-associated fibroblast [11]. Among these, MDSCs are generated in the bone marrow, and in the context of cancer (tumor-bearing hosts), they further migrate to peripheral lymphoid organs and the tumor site [12] and exert immune suppressive activity [13], inhibiting the functions of cytotoxic T cells and natural killer cells [14]. Moreover, the tumor microenvironment in osteosarcoma (OS-TME) houses essential macrophage populations, which can be categorized into two main phenotypes: the classically activated macrophages, often referred to as Type-1 (OS-M1), and the alternative Type-2 macrophages (OS-M2) [15]. Within the OS-TME, the prevailing majority of macrophages primarily display the pro-tumor Type-2 phenotype, lacking anti-tumor functionalities [16]. Osteosarcoma-associated neutrophils also participate in the OS-TME [17]. Initially, neutrophils are responsible for immune attack; however, the OS educates the neutrophils to become tumor-promoting neutrophils [18]. Regulatory T lymphocytes (Tregs) in the immunosuppressive or cold OS-TME also matter, favoring OS progression and metastasis [19]. To effectively treat OS, it is essential to alter the OS-TME.
Traditional intervention approaches, such as surgical resection and chemotherapy, are primary strategies against OS. Cancer immunotherapy has surfaced as an important avenue for the improvement in OS-TME, benefiting the inhibition of metastasis and recurrence of OS [20]. For the therapy associated with the OS-M, researchers divided current immunotherapy for regulating the OS-M into two types: conventional immunotherapeutic and novel engineered cell therapy [21][22]. The conventional approaches encompass the inhibition of recruitment of tumor-promoting OS-M, the impairment of OS-M, and the recovery of OS-M phagocytosis function [23]. Nanotechnology is a significant platform for the therapeutics of modulating the OS-TME. Nanoparticles offer multiple advantages, including the ability to be intricately engineered for the robust and precise activation of T cells before their adoptive transfer [24]. Additionally, Nps can be used to provide the added functionality of immunotherapy [25][26]. Thus, nanoparticles present a promising solution to address the challenges associated with T cell therapy [27][28]. To enhance the accumulation of therapeutics at tumor sites, it is crucial for nanocarriers (NCs) to exhibit prolonged circulation, a characteristic achievable through surface modification with hydrophilic polymers like polyethylene glycol (PEGylation) [29]. Additionally, nanoparticles can also encapsulate agents with a size range of 50–100 nm and can effectively enter parenchymal hepatic cells, stimulating T cell activity [30]. Nanocarriers with a size less than 50 nm can penetrate the cellular barriers, promoting nanoparticle distribution into the bone lesion [31].
Another type of immunotherapy focus is on enhancing the natural ability of adaptive immune cells, particularly cytotoxic T lymphocytes (CTLs) [32]. Adoptive T cell therapy stands as a promising frontier in the realm of cancer treatment [33][34]. Clinical trials have provided compelling evidence of its potential in the context of both adult and pediatric populations, demonstrating the effectiveness of advanced immunotherapy in combatting bone cancer [23][35]. The impact of this therapy has been further underscored by the approval of three notable T cell-based therapeutics—Kymriah, Yescarta, and Breyanzi—by the US FDA [36][37][38]. However, though there has been considerable success in hematologic malignancy treatment, including B cell leukemia and lymphoma treatment, adoptive T cell therapy faces inherent challenges that limit its efficacy in the treatment of many solid tumors including OS [39]. The underlying reason includes the difficulty of T cell infiltration towards the solid tumor because of extensive surrounding stromal cells around OS [40]. To overcome this barrier, one of the approaches is to eliminate the stromal cells with other therapeutics, and nanoparticles can serve as efficient carriers for these therapeutics. Moreover, for future CAR T therapies, it is important to incorporate “on or off switches” that ensure CAR T efficacy in the tumor lesion [41]. Additionally, the serious side effects of CAR T such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) also hinder the success of CAR T [42][43]. Therefore, the combinations of anti-cytokine therapy or synergistic therapeutics to reduce the dose of CAR T will benefit CAR T cell application. In addition, it is reported that a third-generation GD2-CAR exerted effective recognition for the GD2+ sarcoma cell lines in vitro [44]. However, these GD2-CAR T cells were unsuccessful in an in vivo xenograft tumor model [44]. Similarly, it is significant to monitor the HER2 CAR T cell potency using a xenograft or validated model [45]. All finding indicated that the combinational strategies is significant. Another approach enhancing cancer specificity and clinical response is represented by a bispecific CAR T cell molecule [46][47][48].
2. Immunosuppressive Osteosarcoma Microenvironment
Osteosarcoma is known for its high heterogenicity and low tumor immunogenicity. The extensive immune cell infiltration in the OS-TME leads to the formation of a niche for OS proliferation, metastasis, and resistance [17][49]. This immunosuppressive OS-TME is correlated with the presence of MDSCs (Myeloid-Derived Suppressor Cells), OS-Ms (osteosarcoma-associated macrophages), ECs (endothelial cells), and Tregs (regulatory T lymphocytes) [50]. Among them, MDSCs are a heterogeneous population of immune cells that play a crucial role in suppressing the anti-tumor immune response [51]. OS-M refers to tumor promoting macrophages, which, in general, contribute to tumor progression via promoting angiogenesis, tissue remodeling, and immunosuppression. ECs (endothelial cells) are responsible for the supply of nutrients [52][53]. Regulatory T lymphocytes (Tregs) is beneficial for the immune surveillance [19].
2.1. MDSC
As per the brief discussion on MDSCs in the introduction, MDSC is significant component in the OS-TME. Even in the initial stage, MDSC contributes to the pathogenesis of OS through several mechanisms [14]. Firstly, MDSCs hinder T cell migration and reduce T cell viability, making T cell access to the OS more difficult [54]. Furthermore, MDSCs alter T cell fitness by the production of immune-inhibitory molecules like nitric oxide (NO), reactive oxygen species (ROS), and reactive nitrogen species (RNS). Additionally, MDSC reduces T cell-mediated immune responses via the consumption of L-arginine [55]. Lastly, MDSCs consume vital metabolites necessary for T lymphocyte fitness, further compromising the immune response [56]. Additionally, MDSCs can further migrate to peripheral lymphoid organs, resulting in antigen-specific T cell tolerance, and contributing to the metastasis of OS [12]. Firstly, MDSCs play a role in promoting tumor angiogenesis through the secretion of factors like vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP9) [57]. Both of them support the growth of micro-vessels within the tumor and aid the tumor’s expansion [58]. MDSCs also secrete elevated levels of transforming growth factor-beta (TGF-β) and hepatocyte growth factor (HGF) to benefit the growth of OS in other distant organs [59]. The existing TGF-β and HGF induce epithelial–mesenchymal transition (EMT), a process that enhances the tumor’s ability to invade and metastasize [60]. In the metastatic niche, MDSCs secrete a molecule called versican to contribute to the establishment of metastatic tumor growth [61].
2.2. OS-M
Besides MDSCs, in the OS-TME, OS-M functions as a mutineer [62]. In the initial harsh OS-TME characterized by factors such as hypoxia, low pH, elevated glutathione (GSH) levels, and dysregulated kinase systems [63][64], OS cancer cells educate macrophages to adopt tumor-proliferation-supportive roles. Then, as a feedback, the infiltration of OS-M further aggravates the PD-L1 expression in OS, negatively impacting the cytotoxicity of T cells [65][66]. Furthermore, the hypoxic environment in OS-TME promotes tumor angiogenesis [67][68]. In addition, tumor cells also release signals like IL12 and IL4, along with hypoxia-inducible factors HIF-1α and HIF-2α, to maintain OS-M education and support the dysfunction of DCs [69]. OS-M elevates the levels of vascular endothelial growth factor as well as matrix metalloprotease 9 [70]. This facilitates angiogenesis and the formation of a pre-metastatic niche, demonstrating a strong association with osteosarcoma metastasis [71].
2.3. Endothelial Cell
Endothelial cells (ECs) play a role in promoting the acquisition of tumor cell properties, including cell growth, invasion, metastasis, and chemoresistance [72][73]. EC proliferation is associated with nutrient supplies for the OS-TME [74]. It is identified that cyclin-dependent kinase 2 and 5 (Cdk2, Cdk5) serve as key mediators of neo-angiogenesis [75][76]. Additionally, a specific signal named Yin Yang 1 (YY1) protein from osteosarcoma (SaOS) cells plays a crucial role in driving the proliferation of human aortic endothelial cells (HAECs) [76]. In addition to resident endothelial cells, there are circulating ECs. Elevated levels of circulating endothelial cells (CECs) have been found in the peripheral blood of OS patients compared to control groups [77][78]. On the contrary, circulating endothelial progenitor cells (CEPs) are cells derived from the bone, specifically contributing to tumor-associated vasculogenic effects [79]. Additionally, ECs function as both modulators and effectors in the context of OS, contributing to the acceleration of OS exacerbation through the release of von Willebrand factor (VWF) [80].
2.4. Treg
Treg cells represent a dynamic subset of CD4+ T lymphocytes that regulate both normal and aberrant immune system responses [81][82]. Tregs in the TME play critical roles in enabling tumor cells to evade immune surveillance [83]. Some important molecules associated with Tregs will be discerned in this part. For instance, CD39, an ectonucleotidase elevated on Treg cell surfaces, facilitates immunosuppression [84][85]. CD39 converts adenosine triphosphate to adenosine; the subsequent biological binding of adenosine to the A2A receptors (A2AR) and/or A2B receptors (A2BR) has a negative impact on the functions of natural killer and dendritic cells in the TME [85][86][87]. What is worse, adenosine potentiates the expansion of tumor promoting cells, including MDSCs and OS-M2 [87]. Additionally, Tregs secrete perforin and granzymes affecting effector T cells [88][89]. The neuropilin-1 (Nrp1) semaphorin-4a (Sema4a) axis is a newly found factor associated with Tregs in the TME [90]. In addition, CTLA-4 on Tregs causes the direct suppression of the APC function of DCs and hampers the abilities of effector T cells [91]. Thus, the manipulation of Treg function through therapeutic interventions has become a promising strategy.
2.5. OS-Ns
OS-Ns refers to osteosarcoma-related neutrophils [92][93]. OS-Ns has phenotypic heterogeneity and functional versatility. In osteosarcoma, research on TANs is still in its early stages. The lifespan of OS-Ns is longer than that of circulating neutrophils [94][95]. Liu et al. used a meta-analysis to examine the possible correlation between matrix metalloproteinases -9 (MMP-9) mediated by OS-N and a poor prognosis for patients [94][95]. The higher the level of matrix MMP-9 expression, the higher the poor-prognosis risk of patients with OS [94]. Of note, this study faced challenges from another researcher, and thus more studies are essential to validate the prognostic value of MMP9 in OS [94][96]. Neutrophil extracellular traps are web-like chromatin structures formed by the granule proteins and chromatin generated by OS-Ns, which contribute to metastasis, and the underlying mechanism is associated with the DNA receptor coiled-coil domain which includes protein 25 [97]. Another significant chromatin that forms OS-N extracellular traps is peptidylarginine deiminase 4 which overexpressed on OS [98]. Furthermore, the infiltration of neutrophils promotes the translation of hypoxia-related genes, resulting in an increasingly hypoxic microenvironment [99]. A hypoxic TME is not beneficial for the efficacy of anti-cancer therapy [100][101]. It is also deserve to mention that the predominant OS-N in the OS lesion significantly increases the immune escape evasion of OS cells [99].
3. Interactions of Tumor Promoting Cells in the OS-TME
Osteoimmunology is a new term used to describe the immune TME in bone-related disease. The abovementioned immune cells facilitate the evasion of immune attack via immunoediting or other escape mechanisms [102][103]. The OS cell itself is able to downregulate human immunity or achieve immune escape via PDL1/L2 [104], B7-H3 [105], HHLA2 [106], or MHC class II [52][107]. In addition, OS cells release abundant VEGF that interacts with VEGFRs in the endothelial cells (ECs), benefiting angiogenesis and facilitating OS nutrient supply [108].
OS cells release TGF-beta to increase the ratio of Treg in the OS-TME [17]. MDSCs release IL-10 and TNF-alpha, which decrease the activity of cytotoxic T lymphocytes (CTLs) [109]. In the OS-TME, after neo-adjuvant chemotherapy, elevated cytotoxic lymphocyte TILs are associated with a decrease in MDSCs [110]. OS-Ms generally exert pro-tumoral function and show a high correlation with OS aggressiveness and poor prognosis [110][111]. In the OS-TME, OS-M also benefits the formation of neo-vessels via the interaction of the VEGF/VEGF receptor [52]. CTL is responsible for the killing of OS; however, the development of immune surveillance pathways, including the PD-L1/L2-PD1 andCTLA-4 [112], T cell immunoglobulin and mucin-domain-containing-3 (TIM-3) as well as lymphocyte activation gene-3 (LAG-3) [113], ensures the immune escape of OS [62]. Recent preclinical studies have evidenced the TME-promoting role for Tregs in OS [114]. Yoshida. et al. reported that the decrease in Tregs is paralleled by the increase in TILs in the OS-TME [115]. Tregs negatively impact the cytotoxic activity of T cells via TIM-3 as well as LAG-3 [113]. Immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain (TIGIT) on T cells have surfaced as immune targets [116]. All cells actively interact with each other and form an immunosuppressive OS-TME (Figure 1).
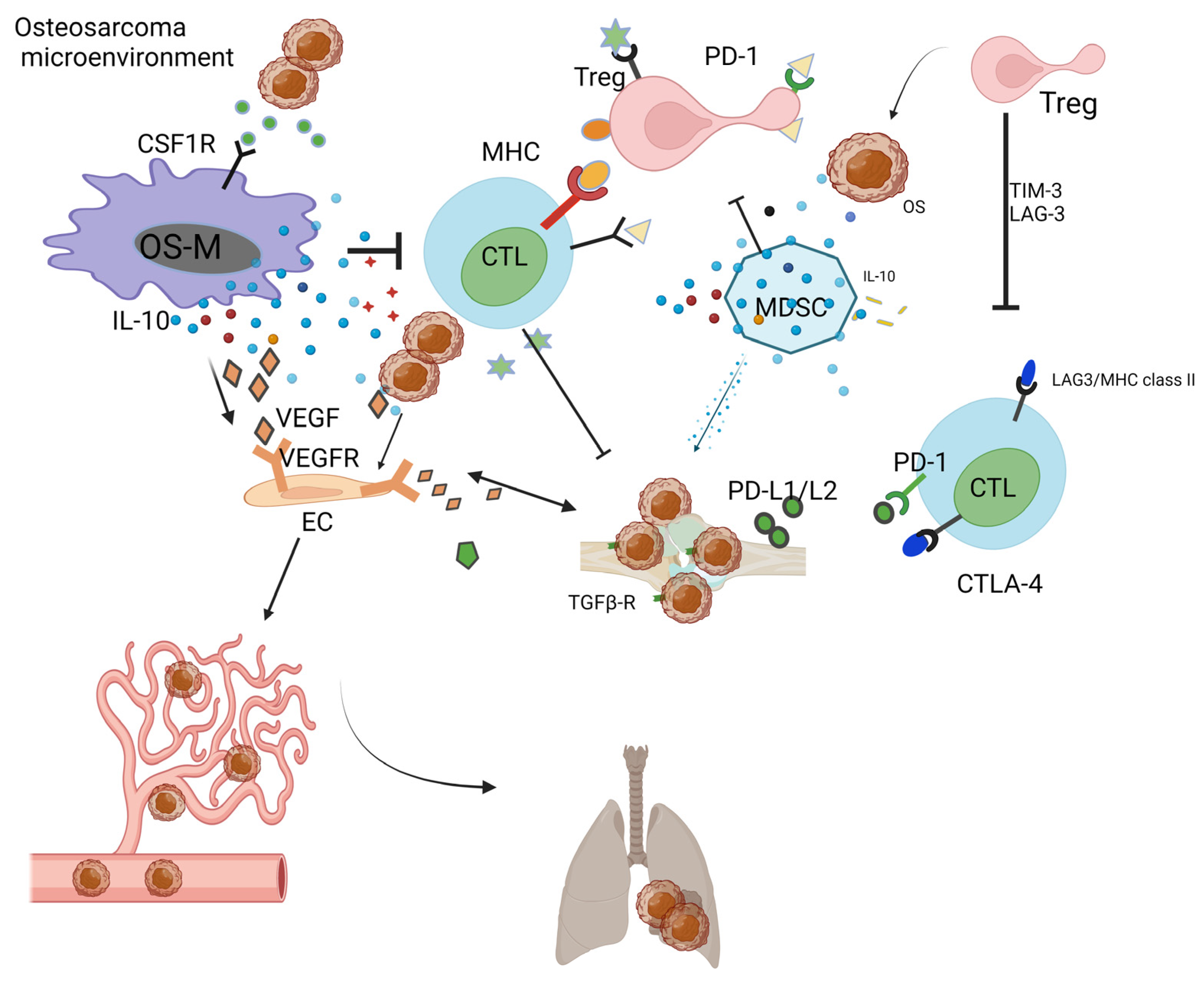
Figure 1. Immunosuppressive OS-TME: Immune-related cells including OS-M, CTL, Treg, MDSC, and OS-N closely work with each other, facilitating the formation of immunosuppressive OS-TME that benefits the proliferation of OS. The MDSC, OS-M, and Treg benefit angiogenesis via VEGF pathways. The OS-TME favors the cancer metastasis process from the primary bone site to the distant lung site.
References
- Jafari, F.; Javdansirat, S.; Sanaie, S.; Naseri, A.; Shamekh, A.; Rostamzadeh, D.; Dolati, S. Osteosarcoma: A comprehensive review of management and treatment strategies. Ann. Diagn. Pathol. 2020, 49, 151654.
- Lawrence, R.C.; Helmick, C.G.; Arnett, F.C.; Deyo, R.A.; Felson, D.T.; Giannini, E.H.; Heyse, S.P.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1998, 41, 778–799.
- Zhao, X.; Wu, Q.; Gong, X.; Liu, J.; Ma, Y. Osteosarcoma: A review of current and future therapeutic approaches. Biomed. Eng. Online 2021, 20, 1–14.
- Lin, Z.; Wu, Z.; Luo, W. Chimeric antigen receptor T-cell therapy: The light of day for osteosarcoma. Cancers 2021, 13, 4469.
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours; John Wiley & Sons: Hoboken, NJ, USA, 2017.
- Stiller, C.; Bielack, S.; Jundt, G.; Steliarova-Foucher, E. Bone tumours in European children and adolescents, 1978–1997. Report from the Automated Childhood Cancer Information System project. Eur. J. Cancer 2006, 42, 2124–2135.
- Tomita, K.; Kawahara, N.; Murakami, H.; Demura, S. Total en bloc spondylectomy for spinal tumors: Improvement of the technique and its associated basic background. J. Orthop. Sci. 2006, 11, 3–12.
- Hillmann, A.; Hoffmann, C.; Gosheger, G.; Krakau, H.; Winkelmann, W. Malignant tumor of the distal part of the femur or the proximal part of the tibia: Endoprosthetic replacement or rotationplasty. Functional outcome and quality-of-life measurements. JBJS 1999, 81, 462–468.
- Hoffmann, C.; Gosheger, G.; Gebert, C.; Jürgens, H.; Winkelmann, W. Functional results and quality of life after treatment of pelvic sarcomas involving the acetabulum. JBJS 2006, 88, 575–582.
- Saeter, G.; Høie, J.; Stenwig, A.E.; Johansson, A.K.; Hannisdal, E.; Solheim, Ø.P. Systemic relapse of patients with osteogenic sarcoma. Prognostic factors for long term survival. Cancer 1995, 75, 1084–1093.
- Labani-Motlagh, A.; Ashja-Mahdavi, M.; Loskog, A. The tumor microenvironment: A milieu hindering and obstructing antitumor immune responses. Front. Immunol. 2020, 11, 940.
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The role of myeloid-derived suppressor cells (MDSC) in cancer progression. Vaccines 2016, 4, 36.
- De Vlaeminck, Y.; González-Rascón, A.; Goyvaerts, C.; Breckpot, K. Cancer-associated myeloid regulatory cells. Front. Immunol. 2016, 7, 113.
- Ling, Z.; Yang, C.; Tan, J.; Dou, C.; Chen, Y. Beyond immunosuppressive effects: Dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell. Mol. Life Sci. 2021, 78, 7161–7183.
- Zając, A.E.; Czarnecka, A.M.; Rutkowski, P. The Role of Macrophages in Sarcoma Tumor Microenvironment and Treatment. Cancers 2023, 15, 5294.
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining tumor-associated macrophage subpopulations and functions in the tumor microenvironment. Front. Immunol. 2020, 11, 1731.
- Nirala, B.K.; Yamamichi, T.; Petrescu, D.I.; Shafin, T.N.; Yustein, J.T. Decoding the Impact of Tumor Microenvironment in Osteosarcoma Progression and Metastasis. Cancers 2023, 15, 5108.
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-associated neutrophils in cancer: Going pro. Cancers 2019, 11, 564.
- Ha, T.-Y. The role of regulatory T cells in cancer. Immune Netw. 2009, 9, 209–235.
- Yu, L.; Zhang, J.; Li, Y. Effects of microenvironment in osteosarcoma on chemoresistance and the promise of immunotherapy as an osteosarcoma therapeutic modality. Front. Immunol. 2022, 13, 871076.
- Payoe, K.S. Identification of Novel Therapeutic Targets in Osteosarcoma for the Development of Nanoparticle Based Drug Delivery Systems. Ph.D. Thesis, Brunel University London, London, UK, 2023.
- Fan, Q.; Zuo, J.; Tian, H.; Huang, C.; Nice, E.C.; Shi, Z.; Kong, Q. Nanoengineering a metal–organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2, 3-dioxygenase and myeloid-derived suppressor cells. J. Exp. Clin. Cancer Res. 2022, 41, 162.
- Lu, Y.; Zhang, J.; Chen, Y.; Kang, Y.; Liao, Z.; He, Y.; Zhang, C. Novel immunotherapies for osteosarcoma. Front. Oncol. 2022, 12, 830546.
- Li, Y.; Rezvani, K.; Rafei, H. Next-generation chimeric antigen receptors for T-and natural killer-cell therapies against cancer. Immunol. Rev. 2023, 320, 217–235.
- Zeng, Z.; Pu, K. Improving cancer immunotherapy by cell membrane-camouflaged nanoparticles. Adv. Funct. Mater. 2020, 30, 2004397.
- Muluh, T.A.; Chen, Z.; Li, Y.; Xiong, K.; Jin, J.; Fu, S.; Wu, J. Enhancing cancer immunotherapy treatment goals by using nanoparticle delivery system. Int. J. Nanomed. 2021, 16, 2389–2404.
- Tang, L.; Zheng, Y.; Melo, M.B.; Mabardi, L.; Castaño, A.P.; Xie, Y.-Q.; Li, N.; Kudchodkar, S.B.; Wong, H.C.; Jeng, E.K. Enhancing T cell therapy through TCR-signaling-responsive nanoparticle drug delivery. Nat. Biotechnol. 2018, 36, 707–716.
- Zheng, C.; Zhang, J.; Chan, H.F.; Hu, H.; Lv, S.; Na, N.; Tao, Y.; Li, M. Engineering nano-therapeutics to boost adoptive cell therapy for cancer treatment. Small Methods 2021, 5, 2001191.
- Attia, M.F.; Anton, N.; Wallyn, J.; Omran, Z.; Vandamme, T.F. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J. Pharm. Pharmacol. 2019, 71, 1185–1198.
- Balakrishnan, P.B.; Sweeney, E.E. Nanoparticles for enhanced adoptive T cell therapies and future perspectives for CNS tumors. Front. Immunol. 2021, 12, 600659.
- Barua, S.; Mitragotri, S. Challenges associated with penetration of nanoparticles across cell and tissue barriers: A review of current status and future prospects. Nano Today 2014, 9, 223–243.
- Durgeau, A.; Virk, Y.; Corgnac, S.; Mami-Chouaib, F. Recent advances in targeting CD8 T-cell immunity for more effective cancer immunotherapy. Front. Immunol. 2018, 9, 14.
- Cerrano, M.; Ruella, M.; Perales, M.-A.; Vitale, C.; Faraci, D.G.; Giaccone, L.; Coscia, M.; Maloy, M.; Sanchez-Escamilla, M.; Elsabah, H. The advent of CAR T-cell therapy for lymphoproliferative neoplasms: Integrating research into clinical practice. Front. Immunol. 2020, 11, 888.
- McKee, M.D.; Fichera, A.; Nishimura, M.I. T cell immunotherapy. Front. Biosci.-Landmark 2007, 12, 919–932.
- Chen, C.; Xie, L.; Ren, T.; Huang, Y.; Xu, J.; Guo, W. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021, 500, 1–10.
- Ittershagen, S.; Ericson, S.; Eldjerou, L.; Shojaee, A.; Bleickardt, E.; Patel, M.; Taran, T.; Anak, O.; Hall, C.; Leung, M. Industry’s giant leap into cellular therapy: Catalyzing chimeric antigen receptor T cell (CAR-T) immunotherapy. Curr. Hematol. Malign. Rep. 2019, 14, 47–55.
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 2018, 378, 439–448.
- Wang, X.; Rivière, I. Manufacturing of CAR-T Cells: The Assembly Line. In Gene and Cellular Immunotherapy for Cancer; Springer: Berlin/Heidelberg, Germany, 2022; pp. 121–139.
- Mirzaei, H.R.; Rodriguez, A.; Shepphird, J.; Brown, C.E.; Badie, B. Chimeric antigen receptors T cell therapy in solid tumor: Challenges and clinical applications. Front. Immunol. 2017, 8, 1850.
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859.
- Melosky, B. Cutaneous Reactions to Tyrosine Kinase Inhibitors. In Skin Diseases in the Immunocompromised; Springer: Berlin/Heidelberg, Germany, 2014; pp. 107–120.
- Sheth, V.S.; Gauthier, J. Taming the beast: CRS and ICANS after CAR T-cell therapy for ALL. Bone Marrow Transplant. 2021, 56, 552–566.
- Wallet, F.; Sesques, P.; Devic, P.; Levrard, M.; Ader, F.; Friggeri, A.; Bachy, E. CAR-T cell: Toxicities issues: Mechanisms and clinical management. Bull. Du Cancer 2021, 108, S117–S127.
- Long, A.H.; Highfill, S.L.; Cui, Y.; Smith, J.P.; Walker, A.J.; Ramakrishna, S.; El-Etriby, R.; Galli, S.; Tsokos, M.G.; Orentas, R.J. Reduction of MDSCs with all-trans retinoic acid improves CAR therapy efficacy for sarcomas. Cancer Immunol. Res. 2016, 4, 869–880.
- Ahmed, N.; Salsman, V.S.; Yvon, E.; Louis, C.U.; Perlaky, L.; Wels, W.S.; Dishop, M.K.; Kleinerman, E.E.; Pule, M.; Rooney, C.M. Immunotherapy for osteosarcoma: Genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 2009, 17, 1779–1787.
- Strohl, W.R.; Naso, M. Bispecific T-cell redirection versus chimeric antigen receptor (CAR)-T cells as approaches to kill cancer cells. Antibodies 2019, 8, 41.
- Slaney, C.Y.; Wang, P.; Darcy, P.K.; Kershaw, M.H. CARs versus BiTEs: A comparison between T cell–redirection strategies for cancer treatment. Cancer Discov. 2018, 8, 924–934.
- Jena, B.; Dotti, G.; Cooper, L.J. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood J. Am. Soc. Hematol. 2010, 116, 1035–1044.
- Somaiah, N.; Conley, A.P.; Parra, E.R.; Lin, H.; Amini, B.; Soto, L.S.; Salazar, R.; Barreto, C.; Chen, H.; Gite, S. Durvalumab plus tremelimumab in advanced or metastatic soft tissue and bone sarcomas: A single-centre phase 2 trial. Lancet Oncol. 2022, 23, 1156–1166.
- Xie, X.; Feng, Y.; Zhang, H.; Su, Q.; Song, T.; Yang, G.; Li, N.; Wei, X.; Li, T.; Qin, X.; et al. Remodeling tumor immunosuppressive microenvironment via a novel bioactive nanovaccines potentiates the efficacy of cancer immunotherapy. Bioact. Mater. 2022, 16, 107–119.
- Wen, L.; Tian-Cong, W.; Dong-Mei, H.; Yue, H.; Ting, F.; Wen-Jie, G.; Qiang, X.J. Carnosic acid enhances the anti-lung cancer effect of cisplatin by inhibiting myeloid-derived suppressor cells. Chin. J. Nat. Med. 2018, 16, 907–915.
- Corre, I.; Verrecchia, F.; Crenn, V.; Redini, F.; Trichet, V. The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells 2020, 9, 976.
- Verhoeven, J.; Baelen, J.; Agrawal, M.; Agostinis, P. Endothelial cell autophagy in homeostasis and cancer. FEBS Lett. 2021, 595, 1497–1511.
- Pramanik, A.; Bhattacharyya, S. Myeloid derived suppressor cells and innate immune system interaction in tumor microenvironment. Life Sci. 2022, 305, 120755.
- Pinton, L. The Crosstalk between Activated T Cells and Myeloid Derived Suppressor Cells: Characterization of Molecular Mechanisms Involved in Immune Suppression. 2014. Available online: https://www.research.unipd.it/handle/11577/3423699 (accessed on 1 April 2014).
- Le Bourgeois, T.; Strauss, L.; Aksoylar, H.-I.; Daneshmandi, S.; Seth, P.; Patsoukis, N.; Boussiotis, V.A. Targeting T cell metabolism for improvement of cancer immunotherapy. Front. Oncol. 2018, 8, 237.
- Rivera, L.B.; Bergers, G. Myeloid cell-driven angiogenesis and immune regulation in tumors. Trends Immunol. 2015, 36, 240.
- Rak, J.W.; St Croix, B.D.; Kerbel, R.S. Consequences of angiogenesis for tumor progression, metastasis and cancer therapy. Anti-Cancer Drugs 1995, 6, 3–18.
- Xue, V.W.; Chung, J.Y.-F.; Córdoba, C.A.G.; Cheung, A.H.-K.; Kang, W.; Lam, E.W.-F.; Leung, K.-T.; To, K.-F.; Lan, H.-Y.; Tang, P.M.-K. Transforming growth factor-β: A multifunctional regulator of cancer immunity. Cancers 2020, 12, 3099.
- Tsubakihara, Y.; Moustakas, A. Epithelial-mesenchymal transition and metastasis under the control of transforming growth factor β. Int. J. Mol. Sci. 2018, 19, 3672.
- Ya, G.; Ren, W.; Qin, R.; He, J.; Zhao, S. Role of myeloid-derived suppressor cells in the formation of pre-metastatic niche. Front. Oncol. 2022, 12, 975261.
- Meftahpour, V.; Aghebati-Maleki, A.; Fotouhi, A.; Safarzadeh, E.; Aghebati-Maleki, L. Prognostic significance and therapeutic potentials of immune checkpoints in osteosarcoma. EXCLI J. 2022, 21, 250.
- Imtiyaz, H.Z.; Simon, M.C. Hypoxia-inducible factors as essential regulators of inflammation. In Diverse Effects of Hypoxia on Tumor Progression; Springer: Berlin/Heidelberg, Germany, 2010; pp. 105–120.
- Balkwill, F. Cancer and the chemokine network. Nat. Rev. Cancer 2004, 4, 540–550.
- Guo, F.; Feng, Y.C.; Zhao, G.; Zhang, R.; Cheng, Z.Z.; Kong, W.N.; Wu, H.L.; Xu, B.; Lv, X.; Ma, X.M. Tumor-Associated CD163(+) M2 Macrophage Infiltration is Highly Associated with PD-L1 Expression in Cervical Cancer. Cancer Manag. Res. 2020, 12, 5831–5843.
- Harada, K.; Dong, X.; Estrella, J.S.; Correa, A.M.; Xu, Y.; Hofstetter, W.L.; Sudo, K.; Onodera, H.; Suzuki, K.; Suzuki, A.; et al. Tumor-associated macrophage infiltration is highly associated with PD-L1 expression in gastric adenocarcinoma. Gastric Cancer 2018, 21, 31–40.
- Pierrevelcin, M.; Fuchs, Q.; Lhermitte, B.; Messé, M.; Guérin, E.; Weingertner, N.; Martin, S.; Lelong-Rebel, I.; Nazon, C.; Dontenwill, M. Focus on hypoxia-related pathways in pediatric osteosarcomas and their druggability. Cells 2020, 9, 1998.
- He, Z.; Zhang, S. Tumor-associated macrophages and their functional transformation in the hypoxic tumor microenvironment. Front. Immunol. 2021, 12, 741305.
- Chen, P.-J.; Chang, C.-H.; Kuo, Y.-L.; Lin, Y.-C. Designing and evaluating a wearable sEMG device for the elderly. In 14th International Conference on ICT, Society, and Human Beings, ICT 2021, 18th International Conference on Web Based Communities and Social Media, WBC 2021 and 13th International Conference on e-Health, EH 2021-Held at the 15th Multi-Conference on Computer Science and Information Systems, MCCSIS 2021; IADIS: Budapest, Hungary, 2021.
- Zhou, J.; Liu, T.; Wang, W. Prognostic significance of matrix metalloproteinase 9 expression in osteosarcoma: A meta-analysis of 16 studies. Medicine 2018, 97, e13051.
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437.
- Sobierajska, K.; Ciszewski, W.M.; Sacewicz-Hofman, I.; Niewiarowska, J. Endothelial cells in the tumor microenvironment. In Tumor Microenvironment: Non-Hematopoietic Cells; Springer: Berlin/Heidelberg, Germany, 2020; pp. 71–86.
- Minami, K.; Ueda, N.; Ishimoto, K.; Tsujiuchi, T. LPA5-mediated signaling induced by endothelial cells and anticancer drug regulates cellular functions of osteosarcoma cells. Exp. Cell Res. 2020, 388, 111813.
- Rodrigues, J.; Sarmento, B.; Pereira, C.L. Osteosarcoma tumor microenvironment: The key for the successful development of biologically relevant 3D in vitro models. In Vitro Models 2022, 1, 5–27.
- Pozo, K.; Bibb, J.A. The emerging role of Cdk5 in cancer. Trends Cancer 2016, 2, 606–618.
- de Nigris, F.; Mancini, F.P.; Schiano, C.; Infante, T.; Zullo, A.; Minucci, P.B.; Al-Omran, M.; Giordano, A.; Napoli, C.J. Osteosarcoma cells induce endothelial cell proliferation during neo-angiogenesis. J. Cell. Physiol. 2013, 228, 846–852.
- Ribatti, D. Morphofunctional Aspects of Tumor Microcirculation; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012.
- Albulescu, R.; Tanase, C. Tumor Angiogenesis: A Target for Renal Cell Carcinoma Therapy. Current Perspectives and Novel Strategies. Recent Pat. Biomark. 2012, 2, 99–106.
- Furuya, M.; Yonemitsu, Y. Cancer neovascularization and proinflammatory microenvironments. Curr. Cancer Drug Targets 2008, 8, 253–265.
- Tawil, N. Studies on Glioblastoma Associated Thrombosis: Impact of Tumor Cell Heterogeneity and Procoagulant Extracellular Vesicles. Ph.D. Thesis, McGill University, Montréal, QC, Canada, 2021.
- Phetsouphanh, C.; Xu, Y.; Zaunders, J. CD4 T cells mediate both positive and negative regulation of the immune response to HIV infection: Complex role of T follicular helper cells and regulatory T cells in pathogenesis. Front. Immunol. 2015, 5, 681.
- Fehérvari, Z.; Sakaguchi, S. CD4+ Tregs and immune control. J. Clin. Investig. 2004, 114, 1209–1217.
- Lucca, L.E.; Dominguez-Villar, M. Modulation of regulatory T cell function and stability by co-inhibitory receptors. Nat. Rev. Immunol. 2020, 20, 680–693.
- Whiteside, T.L. FOXP3+ Treg as a therapeutic target for promoting anti-tumor immunity. Expert Opin. Ther. Targets 2018, 22, 353–363.
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Höpner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood J. Am. Soc. Hematol. 2007, 110, 1225–1232.
- Young, A.; Mittal, D.; Stagg, J.; Smyth, M.J. Targeting cancer-derived adenosine: New therapeutic approaches. Cancer Discov. 2014, 4, 879–888.
- Sek, K.; Mølck, C.; Stewart, G.D.; Kats, L.; Darcy, P.K.; Beavis, P.A. Targeting adenosine receptor signaling in cancer immunotherapy. Int. J. Mol. Sci. 2018, 19, 3837.
- Togashi, Y.; Shitara, K.; Nishikawa, H. Regulatory T cells in cancer immunosuppression—Implications for anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 356–371.
- Raffin, C.; Vo, L.T.; Bluestone, J.A. Treg cell-based therapies: Challenges and perspectives. Nat. Rev. Immunol. 2020, 20, 158–172.
- Delgoffe, G.M.; Woo, S.-R.; Turnis, M.E.; Gravano, D.M.; Guy, C.; Overacre, A.E.; Bettini, M.L.; Vogel, P.; Finkelstein, D.; Bonnevier, J. Stability and function of regulatory T cells is maintained by a neuropilin-1–semaphorin-4a axis. Nature 2013, 501, 252–256.
- Sansom, D.M.; Walker, L.S. The role of CD28 and cytotoxic T-lymphocyte antigen-4 (CTLA-4) in regulatory T-cell biology. Immunol. Rev. 2006, 212, 131–148.
- Yang, S.; Wu, C.; Wang, L.; Shan, D.; Chen, B. Pretreatment inflammatory indexes as prognostic predictors for survival in osteosarcoma patients. Int. J. Clin. Exp. Pathol. 2020, 13, 515.
- Yapar, A.; Tokgöz, M.A.; Yapar, D.; Atalay, İ.B.; Ulucaköy, C.; Güngör, B. Diagnostic and prognostic role of neutrophil/lymphocyte ratio, platelet/lymphocyte ratio, and lymphocyte/monocyte ratio in patients with osteosarcoma. Jt. Dis. Relat. Surg. 2021, 32, 489.
- Li, H.; Zhang, K.; Liu, L.-H.; Ouyang, Y.; Bu, J.; Guo, H.-B.; Xiao, T. A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma. Tumor Biol. 2014, 35, 5487–5491.
- Liu, Y.; Wang, Y.; Teng, Z.; Chen, J.; Li, Y.; Chen, Z.; Li, Z.; Zhang, Z. Matrix metalloproteinase 9 expression and survival of patients with osteosarcoma: A meta-analysis. Eur. J. Cancer Care 2017, 26, e12364.
- Zhang, Q.; Li, J.; Liu, F.; Li, Z. Comments on Li H et al. “A systematic review of matrix metalloproteinase 9 as a biomarker of survival in patients with osteosarcoma”. Tumour Biol. 2015, 36, 5–6.
- Papayannopoulos, V. Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 2018, 18, 134–147.
- Leshner, M.; Wang, S.; Lewis, C.; Zheng, H.; Chen, X.A.; Santy, L.; Wang, Y. PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 2012, 3, 307.
- Fu, Y.; Bao, Q.; Liu, Z.; He, G.; Wen, J.; Liu, Q.; Xu, Y.; Jin, Z.; Zhang, W. Development and validation of a hypoxia-associated prognostic signature related to osteosarcoma metastasis and immune infiltration. Front. Cell Dev. Biol. 2021, 9, 633607.
- Wang, Y.; Wang, Z.; Jia, F.; Xu, Q.; Shu, Z.; Deng, J.; Li, A.; Yu, M.; Yu, Z. CXCR4-guided liposomes regulating hypoxic and immunosuppressive microenvironment for sorafenib-resistant tumor treatment. Bioact. Mater. 2022, 17, 147–161.
- Li, S.; Yue, H.; Wang, S.; Li, X.; Wang, X.; Guo, P.; Ma, G.; Wei, W. Advances of bacteria-based delivery systems for modulating tumor microenvironment. Adv. Drug Deliv. Rev. 2022, 188, 114444.
- Schreiber, R.D.; Old, L.J.; Smyth, M.J. Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science 2011, 331, 1565–1570.
- Teng, M.W.; Galon, J.; Fridman, W.-H.; Smyth, M.J. From mice to humans: Developments in cancer immunoediting. J. Clin. Investig. 2015, 125, 3338–3346.
- Tanaka, K.; Albin, M.J.; Yuan, X.; Yamaura, K.; Habicht, A.; Murayama, T.; Grimm, M.; Waaga, A.M.; Ueno, T.; Padera, R.F.; et al. PDL1 is required for peripheral transplantation tolerance and protection from chronic allograft rejection. J. Immunol. 2007, 179, 5204–5210.
- Castellanos, J.R.; Purvis, I.J.; Labak, C.M.; Guda, M.R.; Tsung, A.J.; Velpula, K.K.; Asuthkar, S. B7-H3 role in the immune landscape of cancer. Am. J. Clin. Exp. Immunol. 2017, 6, 66.
- Mortezaee, K. HHLA2 immune-regulatory roles in cancer. Biomed. Pharmacother. 2023, 162, 114639.
- Xu, W.; Hiếu, T.; Malarkannan, S.; Wang, L. The structure, expression, and multifaceted role of immune-checkpoint protein VISTA as a critical regulator of anti-tumor immunity, autoimmunity, and inflammation. Cell. Mol. Immunol. 2018, 15, 438–446.
- Gardner, V.; Madu, C.O.; Lu, Y. Anti-VEGF therapy in cancer: A double-edged sword. In Physiologic and Pathologic Angiogenesis-Signaling Mechanisms and Targeted Therapy; InTech: Rijeka, Croatia, 2017; pp. 385–410. Available online: https://books.google.co.jp/books?hl=zh-TW&lr=&id=3PiODwAAQBAJ&oi=fnd&pg=PA385&dq=Anti-VEGF+therapy+in+cancer:+A+double-edged+sword.+In+Physiologic+and+Pathologic+Angiogenesis-Signaling+Mechanisms+and+Targeted+Therapy%3B+2017&ots=XEqyI2AyHw&sig=_VD70iZXLFpr8Stt2DnwO9MUwIU&redir_esc=y#v=onepage&q=Anti-VEGF%20therapy%20in%20cancer%3A%20A%20double-edged%20sword.%20In%20Physiologic%20and%20Pathologic%20Angiogenesis-Signaling%20Mechanisms%20and%20Targeted%20Therapy%3B%202017&f=false (accessed on 1 May 2017).
- Li, K.; Shi, H.; Zhang, B.; Ou, X.; Ma, Q.; Chen, Y.; Shu, P.; Li, D.; Wang, Y. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct. Target. Ther. 2021, 6, 362.
- Cascini, C.; Chiodoni, C. The immune landscape of osteosarcoma: Implications for prognosis and treatment response. Cells 2021, 10, 1668.
- Noy, R.; Pollard, J.W. Tumor-associated macrophages: From mechanisms to therapy. Immunity 2014, 41, 49–61.
- Wang, S.-D.; Li, H.-Y.; Li, B.-H.; Xie, T.; Zhu, T.; Sun, L.-L.; Ren, H.-Y.; Ye, Z.-M. The role of CTLA-4 and PD-1 in anti-tumor immune response and their potential efficacy against osteosarcoma. Int. Immunopharmacol. 2016, 38, 81–89.
- Pu, F.; Chen, F.; Zhang, Z.; Qing, X.; Lin, H.; Zhao, L.; Xia, P.; Shao, Z. TIM-3 expression and its association with overall survival in primary osteosarcoma. Oncol. Lett. 2019, 18, 5294–5300.
- Zhu, T.; Han, J.; Yang, L.; Cai, Z.; Sun, W.; Hua, Y.; Xu, J. Immune microenvironment in osteosarcoma: Components, therapeutic strategies and clinical applications. Front. Immunol. 2022, 13, 907550.
- Yoshida, K.; Okamoto, M.; Sasaki, J.; Kuroda, C.; Ishida, H.; Ueda, K.; Ideta, H.; Kamanaka, T.; Sobajima, A.; Takizawa, T.; et al. Anti-PD-1 antibody decreases tumour-infiltrating regulatory T cells. BMC Cancer 2020, 20, 25.
- Kurita, M.; Yoshihara, Y.; Ishiuji, Y.; Chihara, M.; Ishiji, T.; Asahina, A.; Yanaba, K. Expression of T-cell immunoglobulin and immunoreceptor tyrosine-based inhibitory motif domain on CD4+ T cells in patients with atopic dermatitis. J. Dermatol. 2019, 46, 37–42.
More
Information
Subjects:
Orthopedics
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
580
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




