Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by xuejia kang and Version 2 by Rita Xu.
Immunosuppressive elements within the tumor microenvironment are the primary drivers of tumorigenesis and malignant advancement. The presence, as well as the crosstalk between myeloid-derived suppressor cells (MDSCs), osteosarcoma-associated macrophages (OS-Ms), regulatory T cells (Tregs), and endothelial cells (ECs) with osteosarcoma cells cause the poor prognosis of OS.
- osteosarcoma
- suppressive immune environment
- nanoparticles
1. Introduction
Osteosarcoma, the most prevalent malignant bone-related cancer in adolescents, poses a significant treatment challenge [1]. The survival rate for this condition over a five-year period ranges from 60% to 70%, and it is more frequently observed in males and individuals of African American descent [2]. Despite extensive research efforts, 5-year survival rates for osteosarcoma patients have remained around 20% in recent decades [3]. What is worse, the prognosis for patients with metastatic or relapsed osteosarcoma has remained bleak and stagnant over the past 30 years [4]. The current management strategy for primary OS is surgery plus neoadjuvant chemotherapy (preoperative treatments), and the survival rate has been discovered as 35–40% [5][6][5,6]. Rotation-plasty, a well-established method for knee tumor reconstruction, can yield functional and psychological outcomes equal or superior to endoprosthetic reconstruction [7][8][7,8]. However, it presents cosmetic challenges [7][9][7,9]. Axial skeleton sarcoma surgery is complex due to high local recurrence risk and frequent reconstruction complications [7]. About 30% of primary metastatic osteosarcoma patients and over 40% achieving complete remission can be long-term survivors [10]. Lung metastases in advanced OS patients decrease the 5-year survival rate to 5–10%; in this case, the adjuvant combination of chemotherapy and immunotherapy significantly enhances survival rates. Despite this progress, the immunotherapy of OS still requires in-depth investigation.
The tumor microenvironment (TME) is a complex system composed of not only cancer cells, but also various cell types, including immune cells such as myeloid-derived suppressor cells (MDSCs), osteosarcoma-macrophage (OS-M), and stromal cells like the cancer-associated fibroblast [11]. Among these, MDSCs are generated in the bone marrow, and in the context of cancer (tumor-bearing hosts), they further migrate to peripheral lymphoid organs and the tumor site [12] and exert immune suppressive activity [13], inhibiting the functions of cytotoxic T cells and natural killer cells [14]. Moreover, the tumor microenvironment in osteosarcoma (OS-TME) houses essential macrophage populations, which can be categorized into two main phenotypes: the classically activated macrophages, often referred to as Type-1 (OS-M1), and the alternative Type-2 macrophages (OS-M2) [15]. Within the OS-TME, the prevailing majority of macrophages primarily display the pro-tumor Type-2 phenotype, lacking anti-tumor functionalities [16]. Osteosarcoma-associated neutrophils also participate in the OS-TME [17]. Initially, neutrophils are responsible for immune attack; however, the OS educates the neutrophils to become tumor-promoting neutrophils [18]. Regulatory T lymphocytes (Tregs) in the immunosuppressive or cold OS-TME also matter, favoring OS progression and metastasis [19]. To effectively treat OS, it is essential to alter the OS-TME.
Traditional intervention approaches, such as surgical resection and chemotherapy, are primary strategies against OS. Cancer immunotherapy has surfaced as an important avenue for the improvement in OS-TME, benefiting the inhibition of metastasis and recurrence of OS [20]. For the therapy associated with the OS-M, rwesearchers divided current immunotherapy for regulating the OS-M into two types: conventional immunotherapeutic and novel engineered cell therapy [21][22][21,22]. The conventional approaches encompass the inhibition of recruitment of tumor-promoting OS-M, the impairment of OS-M, and the recovery of OS-M phagocytosis function [23]. Nanotechnology is a significant platform for the therapeutics of modulating the OS-TME. Nanoparticles offer multiple advantages, including the ability to be intricately engineered for the robust and precise activation of T cells before their adoptive transfer [24]. Additionally, Nps can be used to provide the added functionality of immunotherapy [25][26][25,26]. Thus, nanoparticles present a promising solution to address the challenges associated with T cell therapy [27][28][27,28]. To enhance the accumulation of therapeutics at tumor sites, it is crucial for nanocarriers (NCs) to exhibit prolonged circulation, a characteristic achievable through surface modification with hydrophilic polymers like polyethylene glycol (PEGylation) [29]. Additionally, nanoparticles can also encapsulate agents with a size range of 50–100 nm and can effectively enter parenchymal hepatic cells, stimulating T cell activity [30]. Nanocarriers with a size less than 50 nm can penetrate the cellular barriers, promoting nanoparticle distribution into the bone lesion [31].
Another type of immunotherapy focus is on enhancing the natural ability of adaptive immune cells, particularly cytotoxic T lymphocytes (CTLs) [32]. Adoptive T cell therapy stands as a promising frontier in the realm of cancer treatment [33][34][33,34]. Clinical trials have provided compelling evidence of its potential in the context of both adult and pediatric populations, demonstrating the effectiveness of advanced immunotherapy in combatting bone cancer [23][35][23,35]. The impact of this therapy has been further underscored by the approval of three notable T cell-based therapeutics—Kymriah, Yescarta, and Breyanzi—by the US FDA [36][37][38][36,37,38]. However, though there has been considerable success in hematologic malignancy treatment, including B cell leukemia and lymphoma treatment, adoptive T cell therapy faces inherent challenges that limit its efficacy in the treatment of many solid tumors including OS [39]. The underlying reason includes the difficulty of T cell infiltration towards the solid tumor because of extensive surrounding stromal cells around OS [40]. To overcome this barrier, one of the approaches is to eliminate the stromal cells with other therapeutics, and nanoparticles can serve as efficient carriers for these therapeutics. Moreover, for future CAR T therapies, it is important to incorporate “on or off switches” that ensure CAR T efficacy in the tumor lesion [41]. Additionally, the serious side effects of CAR T such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS) also hinder the success of CAR T [42][43][42,43]. Therefore, the combinations of anti-cytokine therapy or synergistic therapeutics to reduce the dose of CAR T will benefit CAR T cell application. In addition, it is reported that a third-generation GD2-CAR exerted effective recognition for the GD2+ sarcoma cell lines in vitro [44]. However, these GD2-CAR T cells were unsuccessful in an in vivo xenograft tumor model [44]. Similarly, it is significant to monitor the HER2 CAR T cell potency using a xenograft or validated model [45]. All finding indicated that the combinational strategies is significant. Another approach enhancing cancer specificity and clinical response is represented by a bispecific CAR T cell molecule [46][47][48][46,47,48].
2. Immunosuppressive Osteosarcoma Microenvironment
Osteosarcoma is known for its high heterogenicity and low tumor immunogenicity. The extensive immune cell infiltration in the OS-TME leads to the formation of a niche for OS proliferation, metastasis, and resistance [17][49][17,49]. This immunosuppressive OS-TME is correlated with the presence of MDSCs (Myeloid-Derived Suppressor Cells), OS-Ms (osteosarcoma-associated macrophages), ECs (endothelial cells), and Tregs (regulatory T lymphocytes) [50]. Among them, MDSCs are a heterogeneous population of immune cells that play a crucial role in suppressing the anti-tumor immune response [51]. OS-M refers to tumor promoting macrophages, which, in general, contribute to tumor progression via promoting angiogenesis, tissue remodeling, and immunosuppression. ECs (endothelial cells) are responsible for the supply of nutrients [52][53][52,53]. Regulatory T lymphocytes (Tregs) is beneficial for the immune surveillance [19].2.1. MDSC
As per the brief discussion on MDSCs in the introduction, MDSC is significant component in the OS-TME. Even in the initial stage, MDSC contributes to the pathogenesis of OS through several mechanisms [14]. Firstly, MDSCs hinder T cell migration and reduce T cell viability, making T cell access to the OS more difficult [54]. Furthermore, MDSCs alter T cell fitness by the production of immune-inhibitory molecules like nitric oxide (NO), reactive oxygen species (ROS), and reactive nitrogen species (RNS). Additionally, MDSC reduces T cell-mediated immune responses via the consumption of L-arginine [55]. Lastly, MDSCs consume vital metabolites necessary for T lymphocyte fitness, further compromising the immune response [56]. Additionally, MDSCs can further migrate to peripheral lymphoid organs, resulting in antigen-specific T cell tolerance, and contributing to the metastasis of OS [12]. Firstly, MDSCs play a role in promoting tumor angiogenesis through the secretion of factors like vascular endothelial growth factor (VEGF) and matrix metalloproteinase 9 (MMP9) [57]. Both of them support the growth of micro-vessels within the tumor and aid the tumor’s expansion [58]. MDSCs also secrete elevated levels of transforming growth factor-beta (TGF-β) and hepatocyte growth factor (HGF) to benefit the growth of OS in other distant organs [59]. The existing TGF-β and HGF induce epithelial–mesenchymal transition (EMT), a process that enhances the tumor’s ability to invade and metastasize [60]. In the metastatic niche, MDSCs secrete a molecule called versican to contribute to the establishment of metastatic tumor growth [61].2.2. OS-M
Besides MDSCs, in the OS-TME, OS-M functions as a mutineer [62]. In the initial harsh OS-TME characterized by factors such as hypoxia, low pH, elevated glutathione (GSH) levels, and dysregulated kinase systems [63][64][63,64], OS cancer cells educate macrophages to adopt tumor-proliferation-supportive roles. Then, as a feedback, the infiltration of OS-M further aggravates the PD-L1 expression in OS, negatively impacting the cytotoxicity of T cells [65][66][65,66]. Furthermore, the hypoxic environment in OS-TME promotes tumor angiogenesis [67][68][67,68]. In addition, tumor cells also release signals like IL12 and IL4, along with hypoxia-inducible factors HIF-1α and HIF-2α, to maintain OS-M education and support the dysfunction of DCs [69]. OS-M elevates the levels of vascular endothelial growth factor as well as matrix metalloprotease 9 [70]. This facilitates angiogenesis and the formation of a pre-metastatic niche, demonstrating a strong association with osteosarcoma metastasis [71].2.3. Endothelial Cell
Endothelial cells (ECs) play a role in promoting the acquisition of tumor cell properties, including cell growth, invasion, metastasis, and chemoresistance [72][73][72,73]. EC proliferation is associated with nutrient supplies for the OS-TME [74]. It is identified that cyclin-dependent kinase 2 and 5 (Cdk2, Cdk5) serve as key mediators of neo-angiogenesis [75][76][75,76]. Additionally, a specific signal named Yin Yang 1 (YY1) protein from osteosarcoma (SaOS) cells plays a crucial role in driving the proliferation of human aortic endothelial cells (HAECs) [76]. In addition to resident endothelial cells, there are circulating ECs. Elevated levels of circulating endothelial cells (CECs) have been found in the peripheral blood of OS patients compared to control groups [77][78][77,78]. On the contrary, circulating endothelial progenitor cells (CEPs) are cells derived from the bone, specifically contributing to tumor-associated vasculogenic effects [79]. Additionally, ECs function as both modulators and effectors in the context of OS, contributing to the acceleration of OS exacerbation through the release of von Willebrand factor (VWF) [80].2.4. Treg
Treg cells represent a dynamic subset of CD4+ T lymphocytes that regulate both normal and aberrant immune system responses [81][82][81,82]. Tregs in the TME play critical roles in enabling tumor cells to evade immune surveillance [83]. Some important molecules associated with Tregs will be discerned in this part. For instance, CD39, an ectonucleotidase elevated on Treg cell surfaces, facilitates immunosuppression [84][85][84,85]. CD39 converts adenosine triphosphate to adenosine; the subsequent biological binding of adenosine to the A2A receptors (A2AR) and/or A2B receptors (A2BR) has a negative impact on the functions of natural killer and dendritic cells in the TME [85][86][87][85,86,87]. What is worse, adenosine potentiates the expansion of tumor promoting cells, including MDSCs and OS-M2 [87]. Additionally, Tregs secrete perforin and granzymes affecting effector T cells [88][89][88,89]. The neuropilin-1 (Nrp1) semaphorin-4a (Sema4a) axis is a newly found factor associated with Tregs in the TME [90]. In addition, CTLA-4 on Tregs causes the direct suppression of the APC function of DCs and hampers the abilities of effector T cells [91]. Thus, the manipulation of Treg function through therapeutic interventions has become a promising strategy.2.5. OS-Ns
OS-Ns refers to osteosarcoma-related neutrophils [92][93][92,93]. OS-Ns has phenotypic heterogeneity and functional versatility. In osteosarcoma, research on TANs is still in its early stages. The lifespan of OS-Ns is longer than that of circulating neutrophils [94][95][94,95]. Liu et al. used a meta-analysis to examine the possible correlation between matrix metalloproteinases -9 (MMP-9) mediated by OS-N and a poor prognosis for patients [94][95][94,95]. The higher the level of matrix MMP-9 expression, the higher the poor-prognosis risk of patients with OS [94]. Of note, this study faced challenges from another researcher, and thus more studies are essential to validate the prognostic value of MMP9 in OS [94][96][94,96]. Neutrophil extracellular traps are web-like chromatin structures formed by the granule proteins and chromatin generated by OS-Ns, which contribute to metastasis, and the underlying mechanism is associated with the DNA receptor coiled-coil domain which includes protein 25 [97]. Another significant chromatin that forms OS-N extracellular traps is peptidylarginine deiminase 4 which overexpressed on OS [98]. Furthermore, the infiltration of neutrophils promotes the translation of hypoxia-related genes, resulting in an increasingly hypoxic microenvironment [99]. A hypoxic TME is not beneficial for the efficacy of anti-cancer therapy [100][101][100,101]. It is also deserve to mention that the predominant OS-N in the OS lesion significantly increases the immune escape evasion of OS cells [99].3. Interactions of Tumor Promoting Cells in the OS-TME
Osteoimmunology is a new term used to describe the immune TME in bone-related disease. The abovementioned immune cells facilitate the evasion of immune attack via immunoediting or other escape mechanisms [102][103][102,103]. The OS cell itself is able to downregulate human immunity or achieve immune escape via PDL1/L2 [104], B7-H3 [105], HHLA2 [106], or MHC class II [52][107][52,107]. In addition, OS cells release abundant VEGF that interacts with VEGFRs in the endothelial cells (ECs), benefiting angiogenesis and facilitating OS nutrient supply [108]. OS cells release TGF-beta to increase the ratio of Treg in the OS-TME [17]. MDSCs release IL-10 and TNF-alpha, which decrease the activity of cytotoxic T lymphocytes (CTLs) [109]. In the OS-TME, after neo-adjuvant chemotherapy, elevated cytotoxic lymphocyte TILs are associated with a decrease in MDSCs [110]. OS-Ms generally exert pro-tumoral function and show a high correlation with OS aggressiveness and poor prognosis [110][111][110,111]. In the OS-TME, OS-M also benefits the formation of neo-vessels via the interaction of the VEGF/VEGF receptor [52]. CTL is responsible for the killing of OS; however, the development of immune surveillance pathways, including the PD-L1/L2-PD1 andCTLA-4 [112], T cell immunoglobulin and mucin-domain-containing-3 (TIM-3) as well as lymphocyte activation gene-3 (LAG-3) [113], ensures the immune escape of OS [62]. Recent preclinical studies have evidenced the TME-promoting role for Tregs in OS [114]. Yoshida. et al. reported that the decrease in Tregs is paralleled by the increase in TILs in the OS-TME [115]. Tregs negatively impact the cytotoxic activity of T cells via TIM-3 as well as LAG-3 [113]. Immunoglobulin and tyrosine-based inhibitory motif (ITIM) domain (TIGIT) on T cells have surfaced as immune targets [116]. All cells actively interact with each other and form an immunosuppressive OS-TME (Figure 1).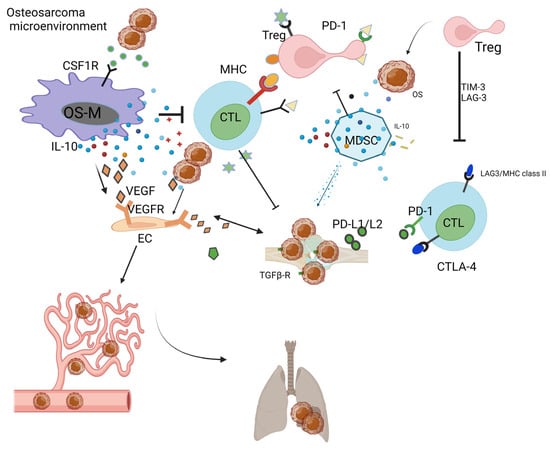
Figure 1. Immunosuppressive OS-TME: Immune-related cells including OS-M, CTL, Treg, MDSC, and OS-N closely work with each other, facilitating the formation of immunosuppressive OS-TME that benefits the proliferation of OS. The MDSC, OS-M, and Treg benefit angiogenesis via VEGF pathways. The OS-TME favors the cancer metastasis process from the primary bone site to the distant lung site.
