Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Zhihong LIU | -- | 2767 | 2024-02-19 12:39:38 | | | |
| 2 | Lindsay Dong | -12 word(s) | 2755 | 2024-02-20 02:01:10 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Liu, Z.; Zhou, T.; Yang, H.; Huang, Z.; Zhang, Y.; Zhang, M. Resourceful Utilization Status for Decommissioned Power Batteries. Encyclopedia. Available online: https://encyclopedia.pub/entry/55170 (accessed on 07 February 2026).
Liu Z, Zhou T, Yang H, Huang Z, Zhang Y, Zhang M. Resourceful Utilization Status for Decommissioned Power Batteries. Encyclopedia. Available at: https://encyclopedia.pub/entry/55170. Accessed February 07, 2026.
Liu, Zhihong, Tuo Zhou, Hairui Yang, Zhong Huang, Yaning Zhang, Man Zhang. "Resourceful Utilization Status for Decommissioned Power Batteries" Encyclopedia, https://encyclopedia.pub/entry/55170 (accessed February 07, 2026).
Liu, Z., Zhou, T., Yang, H., Huang, Z., Zhang, Y., & Zhang, M. (2024, February 19). Resourceful Utilization Status for Decommissioned Power Batteries. In Encyclopedia. https://encyclopedia.pub/entry/55170
Liu, Zhihong, et al. "Resourceful Utilization Status for Decommissioned Power Batteries." Encyclopedia. Web. 19 February, 2024.
Copy Citation
With the rapid development of the new energy vehicle industry, the number of power battery decommissioning is increasing year by year. The recycling of power batteries is of great significance for protecting the ecological environment, improving the efficiency of resource utilization, and ensuring the sustainable and healthy development of the new energy automobile industry.
decommissioned power battery
echelon utilization
pyrometallurgy
hydrometallurgy
direct regeneration
1. Introduction
With the aggravation of global warming and the shortage of oil resources, the promotion of the use of new energy vehicles has become an important measure for the Chinese government to cope with the pressure of energy security and ecological protection [1]. With the power supply of clean energy such as wind energy and solar energy, new energy vehicles have gradually become an important channel for decarbonization in the transportation and energy sectors and have produced good environmental benefits. In addition, they can also be used as portable distributed energy storage systems to store energy, thus playing a vital role in microgrid energy management [2][3].
With the advancement of battery manufacturing technology, the current power batteries that dominate the market no longer contain heavy metal elements such as lead and cadmium, but they still have a variety of pollutants such as carbon black, graphene, and sulfuric acid [4][5]. In recent years, the power battery’s upstream raw material, such as nickel, cobalt, lithium, and other metals, prices continued to rise [6][7]. Taking battery-grade lithium carbonate, for example, at the beginning of 2021, its average price was only 50,000 RMB/ton, and at the beginning of 2022, the price had jumped to 290,000 RMB/ton, a rise of 480%, the highest quoted price even exceeded 300,000 RMB/ton, which makes the power battery recycling have more economic value. Therefore, based on the multi-dimensional considerations of improving power battery utilization efficiency, environmental protection, resource recycling, reducing the risk of the power battery industry supply chain, and realizing the green, low-carbon, and sustainable development of the new energy vehicle industry, it is imperative to effectively recycle and efficiently utilize the decommissioned power battery [8][9].
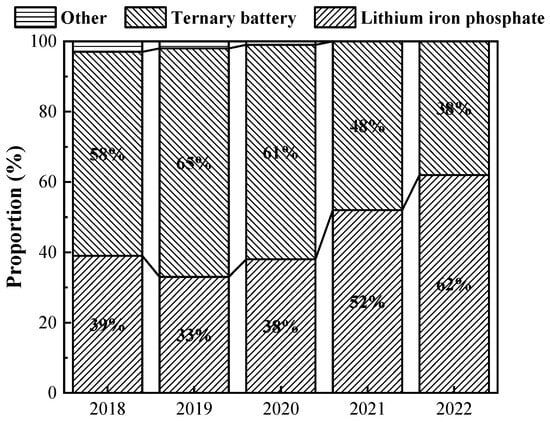
2. Composition of Power Battery
At present, power batteries applied to automobiles can be divided into secondary batteries (including lead–acid batteries, nickel–metal hydride batteries, nickel–chromium batteries, and lithium-ion batteries) and fuel cells [10]. Among them, lithium-ion batteries have better safety, recycling, and repeatable charging performance, as well as higher energy conversion efficiency and more mature manufacturing technology, coupled with China’s lithium resources being more abundant, so lithium-ion batteries have gradually become the mainstream development of power batteries and the first choice for new energy vehicles [11][12][13]. Lithium-ion batteries can be mainly categorized into lithium iron phosphate batteries and ternary batteries. In the early stage of the development of new energy vehicles, lithium iron phosphate batteries occupied a major market share. With the battery energy density included in the subsidy reference, ternary batteries were gradually promoted and used in new energy vehicles. But since 2020, the new energy vehicle subsidy policy has gradually sloped back, and the era of high energy density in exchange for high subsidies has quietly come to an end; lithium iron phosphate batteries, which are better in safety and more cost-effective, have re-entered the field of vision of most automobile enterprises; and the proportion of various types of power battery installations from 2018 to 2022 is shown in Figure 1.

Figure 1. Proportion of installed capacity of various types of power batteries from 2018 to 2022 (data from China Association of Automobile Manufacturers).
Lithium-ion power battery mainly consists of a positive electrode, a diaphragm, a negative electrode, an external protective case, and the electrolyte added in it [14]. The positive electrodes are made of a lithium-containing metal compound coated on a collector and pressed into a sheet, and the lithium-containing compounds used in commercial lithium-ion batteries are usually lithium metal oxides, such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), lithium nickel oxide (LiNiO2), lithium–nickel–cobalt–manganese oxide (LiNixCoyMnzO2, 0 < x, y, z < 1, x + y + z = 1), and lithium iron phosphate (LiFePO4). Negative electrodes are made by coating negative electrode materials (graphite, LTO) onto a copper foil (collector). The electrolyte affects the rate of energy release by controlling the mass flow rate within the cell. The diaphragm is a permeable membrane between the positive and negative electrodes of the battery, mostly made of polymers such as polyethylene or polypropylene, which, on the one hand, prevents physical contact between the electrodes and allows ion transport through the electrolyte and, on the other hand, serves as a safety device; if the battery is overheated, the porous membrane melts and irreversibly seals the electrodes. The external protective case is used to maintain the physical integrity of the battery. The positive and negative electrode materials are the most recyclable parts of a lithium-ion battery.
3. Recycling Methods of Power Battery
The lithium-ion battery recycling route is shown in Figure 2. Power batteries are installed in new energy vehicles after leaving the factory. With the increase in the number of times they are used, the capacity and attenuation performance of the battery gradually decreases. When its performance drops to 70%, it no longer meets the use of electric vehicles, but it can still be used for occasions with low requirements for the performance of the battery, such as energy storage systems, low-speed electric transportation, and at this time, it enters the stage of echelon utilization [15][16]. When the performance of the battery further declines to less than 40%, the value of the echelon utilization is also lost. At this time, the battery can be recycled through the dismantling and recycling methods. According to the above analysis, the method of recycling power batteries can be divided into echelon utilization and recycling.
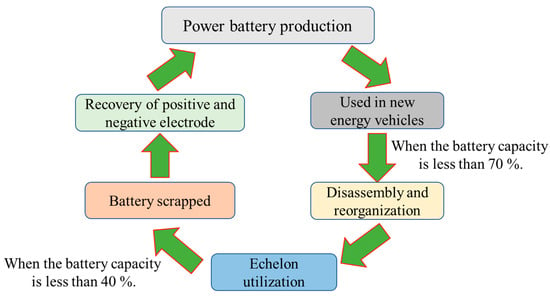
Figure 2. The recycling route of lithium-ion batteries.
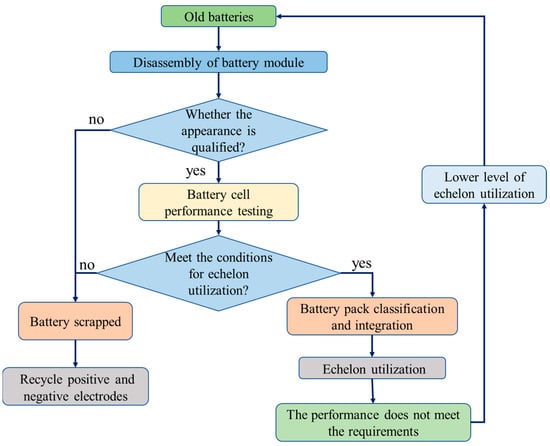
3.1. Echelon Utilization
Generally, power batteries can be used as a power source for new energy vehicles for about 5 years, but their full life cycle from the beginning of use to the complete depletion of energy is about 20 years, which means that vehicle power batteries still have a residual life of about 15 years after scrapping. The echelon utilization is to use the end-of-life vehicle power batteries for other fields with lower requirements on battery performance, the process of which is shown in Figure 3 [17] and usually includes steps such as battery pack disassembly, battery sieving, battery restructuring, and battery module system integration. The decommissioned power battery module needs to be tested twice after disassembly. For the first time, the appearance inspection is used to judge whether the appearance of the battery is qualified, and the battery with bulge, leakage, and deformation is eliminated. The second is the performance test to determine whether the basic performance of the battery meets the requirements and to eliminate the battery with a capacity lower than 40% of the original capacity, abnormal terminal voltage, or excessive internal resistance. The batteries that are qualified in the two tests are classified and integrated to achieve echelon utilization. The unqualified batteries can only be recycled via regenerative utilization.

Figure 3. Processes of the echelon utilization for power batteries [17].
Disassembling is the first step of power battery recycling, and the disassembling method and disassembling efficiency directly affect the echelon utilization. Wegener et al. [18] manually disassembled the hybrid powertrain of an Audi Q5 automobile, derived a disassembly sequence based on a prioritized relationship matrix, and illustrated the disassembly. Some scholars have attempted to introduce automated techniques to battery disassembly.
The state of charge (SOC) and state of health (SOH) of the battery are key factors in determining the occasions for echelon utilization [19]. Therefore, after completing the battery pack disassembly, the disassembled battery cells need to be evaluated for SOC and SOH. SOC is a hidden state quantity, which is difficult to obtain directly from the sensor and can only be estimated based on the parameters of the external characteristics of the battery (terminal voltage, current, temperature). The traditional methods for estimating the SOC of a battery include the open-circuit voltage method [20][21], the internal resistance method [22][23], and the ampere-time integration method [24][25]. With the continuous development of related technologies, the estimation methods and accuracy of the SOC are constantly being revolutionized.
The estimation methods of SOH state can be divided into two kinds: offline estimation method and online estimation method. Among them, offline estimation has the advantages of simplicity and low computation, but it demands strict requirements on the test environment, and the experiments are too time-consuming. In recent years, researchers have proposed some online estimation methods based on offline estimation, such as the slip film observer method [26], voltage curve fitting method [27][28], neural network method [29], and fuzzy logic inference method [30][31], which can realize real-time online estimation of SOH. Nevertheless, SOH evaluation is still difficult due to the many factors affecting the SOH state, including ambient temperature, charge/discharge multiplier, depth of discharge, and charge/discharge cycle, and SOH is a highly nonlinear time-varying system [32].
3.2. Regenerative Utilization
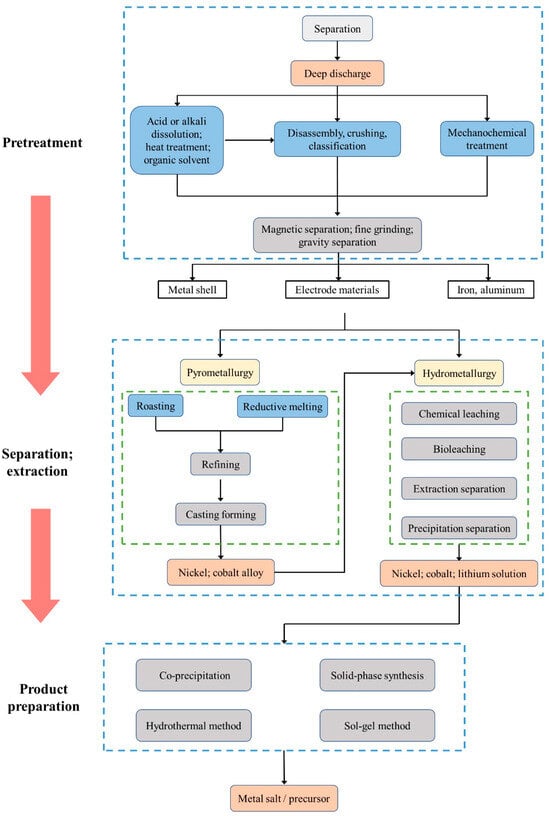
Another resource utilization path for decommissioned power batteries is regeneration, where the batteries that cannot be utilized in the echelon are scrapped, and the valuable metals are refined for the production of new power batteries. Among them, the positive electrode of power batteries contains abundant lithium, cobalt, nickel, manganese, and other metal elements, which have high recycling value and are the core of recycling and reutilization of decommissioned power batteries [33]. Scholars have conducted a lot of research on the regeneration of decommissioned power batteries and summarized the main processes, which mainly include pretreatment, separation and extraction, and product preparation, as shown in Figure 4.

Figure 4. Recycling process of decommissioned power battery [34].
3.2.1. Pretreatment Process
The purpose of pretreatment is to effectively separate the components in the battery and enrich the valuable metal elements, which mainly includes deep discharge, disassembly, classification, crushing and screening, separation, and mechanochemical treatment.
Lithium-ion batteries have a high energy density. At the start of recycling, the batteries still have residual power, so the first step in pre-treatment is to deeply discharge the batteries to avoid spontaneous combustion or explosion during the pre-treatment process [35]. Common discharge methods include immersion, resistance, and perforated discharge.
Disassembly and classification are the key link to realize the regeneration of decommissioned power batteries. Initially, the disassembly and classification were manually realized, and the disassembly efficiency was low. With the development of mechanical and automation technology, researchers have invented numerous devices and systems for the automatic disassembly of power batteries [36].
The disassembled and classified power batteries are crushed; then, physical sorting methods (re-election, electrical, and magnetic separation) can be used to realize the further separation and recovery of different component materials. The crushing process produces exhaust gases, liquids, and residues that are hazardous to human health [37]. Scholars have attempted to improve treatment to avoid the release of toxic and harmful gases.
Crushing and sieving are difficult to dislodge the positive and negative electrode materials from the aluminum and copper foils, so further separation and extraction is required, and the main methods are alkaline dissolution [38], heat treatment [39], organic solvent [40] and ultrasonic treatment [41]. The alkaline dissolution method takes advantage of that the positive electrode material can be stabilized in alkaline conditions, and the aluminum foil will be completely dissolved to make the positive electrode material off. The method is more reported and widely used, so the study will not be introduced in detail. The small safety factor is the main drawback of this method, as the operation requires a high concentration of alkaline solution. The heat treatment method uses high temperatures to volatilize or pyrolyze the binder to separate the positive material from the aluminum foil.
3.2.2. Separation and Extraction
The pretreatment process realizes the enrichment of valuable metal components, but further recovery of valuable metals such as cobalt, lithium, and nickel requires further separation and extraction of the pretreated products. Pyrometallurgy and hydrometallurgy are the most common separation and purification methods at present.
- (1)
-
Pyrometallurgy
Pyrometallurgy refers to the removal of the organic binder in the electrode material with the help of high temperature, and at the same time, the metal and its compounds therein undergo a series of chemical reactions, and finally, the extraction of the target metal material is realized through subsequent separation [42]. The pyrometallurgical process is widely studied because of its simple operation, good adaptability of raw materials, and easy realization of large-scale production.
- (2)
-
Hydrometallurgy
Hydrometallurgy refers to the use of the redox properties of acid or alkali solution to transfer the solid metal in the electrode material into the solution, and then the metal ions in the solution are separated via solvent extraction, chemical precipitation, and other techniques. The hydrometallurgical process is usually divided into two steps: leaching, and separation and purification. Among them, leaching plays a decisive role in the efficiency of metal dissolution, while separation and purification are essential for the production of high-purity materials. According to the type of solvent used for leaching, leaching can be categorized into alkali leaching, acid leaching, and bioleaching.
3.2.3. Product Preparation
The products of pyrometallurgy can be used to produce cobalt-nickel alloys through refining and casting. The products of hydrometallurgy can be used to prepare single-metal salts and to resynthesize battery materials. When preparing single metal salts, nickel, cobalt, and lithium salts need to be separated from the leach solution and further prepared into cobalt sulfate, cobalt chloride, nickel sulfate, nickel carbonate, lithium carbonate, and other products. Resynthesizing battery materials refers to the preparation of positive material precursors by adjusting the pH value and the ratio of metal components after removing the impurity metals from the leach solution, including co-precipitation [43][44], sol-gel [45], high-temperature solid-phase synthesis [46], and hydrothermal methods [47].
3.3. Direct Regeneration of Positive Electrode Materials
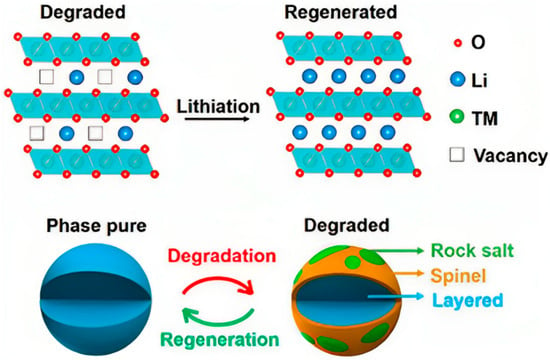
The direct regeneration of positive electrode materials improves the electrochemical performance by adding lithium to the decommissioned positive electrode materials without destroying their crystal structure [48][49]. Compared with other recycling technologies, direct regeneration has the outstanding advantages of a simple process, low cost, and environmental friendliness, and the regenerated positive electrode material can be reused, so it has been developed rapidly. Lithium depletion is the main reason for the failure of lithium-ion battery’s positive electrode materials. The purpose of direct regeneration technology is to combine different lithium replenishment technologies with heat treatment, thus realizing the direct repair of used batteries, including direct solid-state calcination, hydrothermal regeneration, molten salt lithiation, and electrochemical lithium replenishment. Figure 5 shows the direct regeneration of waste ternary materials via lithium supplementation.

Figure 5. Direct regeneration of waste ternary materials via lithium supplementation [50].
Direct solid-state calcination is the most widely used direct regeneration method, especially for batteries with single-component positive electrode materials such as LiCoO2, LiFePO4, and NCM. Chi et al. [51] directly utilized Li2CO3 present on the surface of the waste positive electrode material as a lithium source, placed it together with the positive electrode material to be regenerated in an oxygen atmosphere, and calcined them for 12 h at a temperature of 850 °C to achieve the regeneration purpose.
The hydrothermal regeneration method is to uniformly distribute the lithium source in the aqueous solution so that it is in direct contact with the positive electrode material, thereby supplementing lithium for the failed positive electrode material.
4. Conclusions
The echelon utilization is an important method for the resource utilization of decommissioned power batteries. Restricted by technology and cost, it is difficult to accurately assess the SOH and SOC of the battery. It is the main development direction of the echelon utilization to improve the manufacturing technology of the power battery and reduce the cost of pre-processing. In addition, the development of new methods for rapid and safe assessment of SOH and SOC is also the focus of future researchers.
Regenerative utilization is the main method of resource utilization of decommissioned power batteries. Pre-treatment is the key to the regenerative utilization of power batteries, in which safety, fast, deep discharge, and efficient separation of battery components are the main challenges, and the combination of multiple separation methods is an inevitable choice for the battery pre-treatment link. Pyrometallurgy and hydrometallurgy are the main methods of separation and extraction. Compared with pyrometallurgy, hydrometallurgy has lower energy consumption and less pollution, but its high cost limits its large-scale industrial application. Seeking cheap and efficient leaching agents is the focus of future research.
The direct regeneration of positive electrode materials is the most promising recycling method for decommissioned power batteries. Compositional diversity and uncertainty in the degree of failure are the main factors hindering the direct regeneration of positive electrode materials. Developing suitable pretreatment processes to ensure the extraction of higher purity positive electrode materials without destroying the original crystal structure, as well as exploring how to accurately and rapidly determine the lack of lithium in the waste positive electrode materials, are the future directions for the direct regeneration of positive electrode materials.
References
- Fu, L. Research on the Cooperation Mode between New Energy Vehicle Enterprises and Echelon Utilization Enterprises under the Perspective of Power Battery Recycling and Utilization; Harbin University of Science and Technology: Harbin, China, 2023.
- Chen, Y.; Dou, A.; Zhang, Y. A review of recycling status of decommissioned lithium batteries. Front. Mater. 2021, 8, 634667.
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A systematic review of battery recycling technologies: Advances, challenges, and future prospects. Energies 2023, 16, 6571.
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium-ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308.
- Camargos, P.H.; dos Santos, P.H.J.; dos Santos, I.R.; Ribeiro, G.S.; Caetano, R.E. Perspectives on Li-ion battery categories for electric vehicle applications: A review of state of the art. Int. J. Energy Res. 2022, 46, 19258–19268.
- Barman, P.; Dutta, L.; Azzopardi, B. Electric vehicle battery supply chain and critical materials: A brief survey of state of the art. Energies 2023, 16, 3369.
- Sharmili, N.; Nagi, R.; Wang, P. A review of research in the Li-ion battery production and reverse supply chains. J. Energy Storage 2023, 68, 107622.
- Zante, G.; Braun, A.; Masmoudi, A.; Barillon, R.; Trebouet, D.; Boltoeva, M. Solvent extraction fractionation of manganese, cobalt, nickel and lithium using ionic liquids and deep eutectic solvents. Miner. Eng. 2020, 156, 106512.
- Maddukuri, S.; Malka, D.; Chae, M.S.; Elias, Y.; Luski, S.; Aurbach, D. On the challenge of large energy storage by electrochemical devices. Electrochim. Acta 2020, 354, 136771.
- Xu, Z.; Tian, Y.; Li, J.; Wu, Y.; Huang, J. Development of electric vehicle power battery and its temperature management. Auto Electr. Parts 2018, 54, 1–3.
- Lai, X.; Gu, H.; Chen, Q.; Tang, X.; Zhou, Y.; Gao, F.; Han, X.; Guo, Y.; Bhagat, R.; Zheng, Y. Investigating greenhouse gas emissions and environmental impacts from the production of lithium-ion batteries in China. J. Clean. Prod. 2022, 372, 133756.
- Wang, S.; Yu, J. A comparative life cycle assessment on lithium-ion battery: Case study on electric vehicle battery in China considering battery evolution. Waste Manag. Res. 2021, 39, 156–164.
- Wang, Y.; Yu, Y.; Huang, K.; Tang, B. From the perspective of battery production: Energy-Environment-Economy (3E) analysis of lithium-ion batteries in China. Sustainability 2019, 11, 6941.
- Liu, Y. Structure & Properties Regeneration of Layered Oxides Cathode in Spent Lithium-Ion Batteries; Zhejiang University: Hangzhou, China, 2023.
- Chen, H.; Zhang, T.; Gao, Q.; Han, Z.; Jin, Y.; Li, L.; Yang, K.; Xu, Y.; Liu, X.; Xu, X.; et al. Assessment and management of health status in full life cycle of echelon utilization for retired power lithium batteries. J. Clean. Prod. 2022, 379, 134583.
- Wang, N.; Garg, A.; Su, S.; Mou, J.; Gao, L.; Li, W. Echelon utilization of retired power lithium-ion batteries: Challenges and prospects. Batteries 2022, 8, 96.
- Gao, S.; Zhu, H.; Liu, Z.; Zhao, J.; Bi, H. Reutilization grouping of retired electric vehicle battery based on K-means clustering. Chin. J. Power Sources 2020, 44, 1479–1482+1513. Chin. J. Power Source 2020, 44, 1479–1482+1513.
- Wegener, K.; Andrew, S.; Raatz, A.; Droder, K.; Herrmann, C. Disassembly of electric vehicle batteries using the example of the audi Q5 hybrid system. Procedia CIRP 2014, 23, 155–160.
- Liang, S. Decline Mode Classification and Performance Evaluation of Electric Vehicle Power Battery; Harbin Institute of Technology: Harbin, China, 2016.
- Ma, J.; Zhu, D.; Fang, Y.; Han, Y.; Li, Y. A research on the performance prediction of valve-regulated lead acid battery for BSG hybrid electrical vehicle. Automot. Eng. 2008, 3, 219–221+226.
- Campestrini, C.; Kosch, S.; Jossen, A. Influence of change in open circuit voltage on the state of charge estimation with an extended Kalman filter. J. Energy Storage 2017, 12, 149–156.
- Li, Z.; Zhao, Y. EV battery management system and accurate estimation of SOC. Chin. J. Power Source 2016, 40, 1090–1093.
- Pan, D.; Guo, H.; Tang, S.; Li, X.; Wang, Z.; Peng, W.; Wang, J.; Yan, G. Evaluating the accuracy of electro-thermal coupling model in lithium-ion battery via altering internal resistance acquisition methods. J. Power Source 2020, 463, 228174.
- Guo, H.; Wang, Z.; Li, Y.; Wang, D.; Wang, G. State of charge and parameters estimation for Lithium-ion battery using dual adaptive unscented Kalman filter. In Proceedings of the 29th Chinese Control and Decision Conference (CCDC), Chongqing, China, 28–30 May 2017; pp. 4962–4966.
- Xiao, Z.; Wu, S. Discharge curve-based formation of retired power batteries for secondary use. Int. J. Low-Carbon Technol. 2021, 16, 790–797.
- Kim, I.-S. A technique for estimating the state of health of lithium batteries through a dual-sliding-mode observer. IEEE Trans. Power Electron. 2009, 25, 1013–1022.
- Liu, X. The Estimating of Lithium-Ion Battery Model and SOH of Electric Vehicle; Jilin University: Changchun, China, 2014.
- Lee, S.; Lee, D. Voltage relaxation curve-based state of charge estimation method with reduced measurement time. Electron. Lett. 2023, 59, 12990.
- Andre, D.; Nuhic, A.; Soczka-Guth, T.; Sauer, D.U. Comparative study of a structured neural network and an extended Kalman filter for state of health determination of lithium-ion batteries in hybrid electricvehicles. Eng. Appl. Artif. Intell. 2013, 26, 951–961.
- Zenati, A.; Desprez, P.; Razik, H. Estimation of the SOC and the SOH of Li-ion batteries by combining impedance measurements with the fuzzy logic inference. In Proceedings of the IECON 2010-36th Annual Conference on IEEE Industrial Electronics Society, Glendale, AZ, USA, 7–10 November 2010; pp. 1773–1778.
- Ben Lazreg, M.; Jemmali, S.; Manai, B.; Hamouda, M. Enhanced EKF and SVSF for state of charge estimation of Li-ion battery in electric vehicle using a fuzzy parameters model. IET Electr. Syst. Transp. 2022, 12, 315–329.
- Zhang, Q.C.; Li, X.Z.; Du, Z.C.; Liao, Q.Q. Aging performance characterization and state-of-health assessment of retired lithium-ion battery modules. J. Energy Storage 2021, 40, 102743.
- Bai, Y.; Li, M.; Jafta, C.; Dai, Q.; Essehli, R.; Polzin, B.J.; Belharouak, I. Direct recycling and remanufacturing of anode scraps. Sustain. Mater. Technol. 2023, 35, e00542.
- Liu, H. Research on the Recycling System of Waste Power Batteries of New Energy Vehicles; Shanghai Polytechnic University: Shanghai, China, 2022.
- Li, J. Study on the Physical Separation and Recovery Process of Spent Lithium Ion Power Batteries; General Research Institute for Nonferrous Metals: Beijing, China, 2018.
- Zhou, L.; Garg, A.; Zheng, J.; Gao, L.; Oh, K.Y. Battery pack recycling challenges for the year 2030: Recommended solutions based on intelligent robotics for safe and efficient disassembly, residual energy detection, and secondary utilization. Energy Storage 2021, 3, e190.
- Horai, K.; Shibata, J.; Murayama, N.; Koyanaka, S.; Niinae, M. Recycling Technology for Lithium Ion Battery by Crushing and Classification, and Hydrometallurgical Process. J. Jpn. Inst. Met. Mater. 2014, 78, 250–257.
- He, X. Efficient Leaching of Valuable Metals from LiCoO2 Cathode Material of Spent Lithium-Ion Batteries with Citric Acid Reducing System; Shanghai Polytechnic University: Shanghai, China, 2018.
- Li, L.; Lu, J.; Ren, Y.; Zhang, X.; Chen, R.; Wu, F.; Amine, K. Ascorbic-acid-assisted recovery of cobalt and lithium from spent Li-ion batteries. J. Power Source 2012, 218, 21–27.
- Tran, M.K.; Rodrigues, M.-T.F.; Kato, K.; Babu, G.; Ajayan, P.M. Deep eutectic solvents for cathode recycling of Li-ion batteries. Nat. Energy 2019, 4, 339–345.
- Li, J.; Zhao, R.; He, X.; Liu, H. Preparation of LiCoO2 cathode materials from spent lithium–ion batteries. Ionics 2009, 15, 111–113.
- Qu, G.; Yang, J.; Wang, H.; Ran, Y.; Li, B.; Wei, Y. Applicability of the reduction smelting recycling process to different types of spent lithium-ion batteries cathode materials. Waste Manag. 2023, 166, 222–232.
- Li, L.; Bian, Y.; Zhang, X.; Guan, Y.; Fan, E.; Wu, F.; Chen, R. Process for recycling mixed-cathode materials from spent lithium-ion batteries and kinetics of leaching. Waste Manag. 2018, 71, 362–371.
- Li, G.; Liu, X.; Zhao, Y.; Shao, Z. The preparation and properties research of lithium-rich LiO2 battery cathode materials. Int. J. Electrochem. Sci. 2018, 13, 7321–7334.
- Zhang, A. Study on Recycle and Reuse of Ternary Cathode Materials from Waste Lithium-Ion Batteries; Harbin Institute of Technology: Harbin, China, 2018.
- Lv, W.; Wang, Z.; Cao, H.; Sun, Y.; Zhang, Y.; Sun, Z. A critical review and analysis on the recycling of spent lithium-ion batteries. ACS Sustain. Chem. Eng. 2018, 6, 1504–1521.
- Xie, J. Hydrothermal Synthesis of LiNi0.9Co0.1O2 and LiNi1/3Co1/3Mn1/3O2 for Lithium Ion Batteries; Ocean University of China: Jinan, China, 2011.
- Mukai, K.; Uyama, T.; Nonaka, T. Thermal behavior of Li1+xO4 and a proof of concept for sustainable batteries. ACS Appl. Mater. Interfaces 2021, 13, 42791–42802.
- Li, X.; Zhang, J.; Song, D.; Song, J.; Zhang, L. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries. J. Power Source 2017, 345, 78–84.
- Shi, Y.; Chen, G.; Liu, F.; Yue, X.; Chen, Z. Resolving the compositional and structural defects of degraded LiNixCoyMnzO2 particles to directly regenerate high-performance lithium-ion battery cathodes. ACS Energy Lett. 2018, 3, 1683–1692.
- Chi, Z.; Li, J.; Wang, L.; Li, T.; Wang, Y.; Zhang, Y.; Tao, S.; Zhang, M.; Xiao, Y.; Chen, Y. Direct regeneration method of spent LiNi1/3Co1/3Mn1/3O2 cathode materials via surface lithium residues. Green Chem. 2021, 23, 9099–9108.
More
Information
Subjects:
Engineering, Environmental
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
762
Revisions:
2 times
(View History)
Update Date:
20 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




