Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Zhihong LIU and Version 2 by Lindsay Dong.
With the rapid development of the new energy vehicle industry, the number of power battery decommissioning is increasing year by year. The recycling of power batteries is of great significance for protecting the ecological environment, improving the efficiency of resource utilization, and ensuring the sustainable and healthy development of the new energy automobile industry.
- decommissioned power battery
- echelon utilization
- pyrometallurgy
- hydrometallurgy
- direct regeneration
1. Introduction
With the aggravation of global warming and the shortage of oil resources, the promotion of the use of new energy vehicles has become an important measure for the Chinese government to cope with the pressure of energy security and ecological protection [1]. With the power supply of clean energy such as wind energy and solar energy, new energy vehicles have gradually become an important channel for decarbonization in the transportation and energy sectors and have produced good environmental benefits. In addition, they can also be used as portable distributed energy storage systems to store energy, thus playing a vital role in microgrid energy management [2][3][2,3].
With the advancement of battery manufacturing technology, the current power batteries that dominate the market no longer contain heavy metal elements such as lead and cadmium, but they still have a variety of pollutants such as carbon black, graphene, and sulfuric acid [4][5][9,10]. In recent years, the power battery’s upstream raw material, such as nickel, cobalt, lithium, and other metals, prices continued to rise [6][7][11,12]. Taking battery-grade lithium carbonate, for example, at the beginning of 2021, its average price was only 50,000 RMB/ton, and at the beginning of 2022, the price had jumped to 290,000 RMB/ton, a rise of 480%, the highest quoted price even exceeded 300,000 RMB/ton, which makes the power battery recycling have more economic value. Therefore, based on the multi-dimensional considerations of improving power battery utilization efficiency, environmental protection, resource recycling, reducing the risk of the power battery industry supply chain, and realizing the green, low-carbon, and sustainable development of the new energy vehicle industry, it is imperative to effectively recycle and efficiently utilize the decommissioned power battery [8][9][13,14].
 Lithium-ion power battery mainly consists of a positive electrode, a diaphragm, a negative electrode, an external protective case, and the electrolyte added in it [14][19]. The positive electrodes are made of a lithium-containing metal compound coated on a collector and pressed into a sheet, and the lithium-containing compounds used in commercial lithium-ion batteries are usually lithium metal oxides, such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), lithium nickel oxide (LiNiO2), lithium–nickel–cobalt–manganese oxide (LiNixCoyMnzO2, 0 < x, y, z < 1, x + y + z = 1), and lithium iron phosphate (LiFePO4). Negative electrodes are made by coating negative electrode materials (graphite, LTO) onto a copper foil (collector). The electrolyte affects the rate of energy release by controlling the mass flow rate within the cell. The diaphragm is a permeable membrane between the positive and negative electrodes of the battery, mostly made of polymers such as polyethylene or polypropylene, which, on the one hand, prevents physical contact between the electrodes and allows ion transport through the electrolyte and, on the other hand, serves as a safety device; if the battery is overheated, the porous membrane melts and irreversibly seals the electrodes. The external protective case is used to maintain the physical integrity of the battery. The positive and negative electrode materials are the most recyclable parts of a lithium-ion battery.
Lithium-ion power battery mainly consists of a positive electrode, a diaphragm, a negative electrode, an external protective case, and the electrolyte added in it [14][19]. The positive electrodes are made of a lithium-containing metal compound coated on a collector and pressed into a sheet, and the lithium-containing compounds used in commercial lithium-ion batteries are usually lithium metal oxides, such as lithium cobalt oxide (LiCoO2), lithium manganese oxide (LiMn2O4), lithium nickel oxide (LiNiO2), lithium–nickel–cobalt–manganese oxide (LiNixCoyMnzO2, 0 < x, y, z < 1, x + y + z = 1), and lithium iron phosphate (LiFePO4). Negative electrodes are made by coating negative electrode materials (graphite, LTO) onto a copper foil (collector). The electrolyte affects the rate of energy release by controlling the mass flow rate within the cell. The diaphragm is a permeable membrane between the positive and negative electrodes of the battery, mostly made of polymers such as polyethylene or polypropylene, which, on the one hand, prevents physical contact between the electrodes and allows ion transport through the electrolyte and, on the other hand, serves as a safety device; if the battery is overheated, the porous membrane melts and irreversibly seals the electrodes. The external protective case is used to maintain the physical integrity of the battery. The positive and negative electrode materials are the most recyclable parts of a lithium-ion battery.
2. Composition of Power Battery
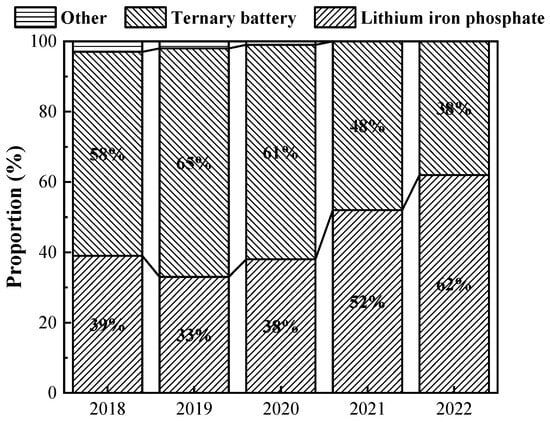
At present, power batteries applied to automobiles can be divided into secondary batteries (including lead–acid batteries, nickel–metal hydride batteries, nickel–chromium batteries, and lithium-ion batteries) and fuel cells [10][15]. Among them, lithium-ion batteries have better safety, recycling, and repeatable charging performance, as well as higher energy conversion efficiency and more mature manufacturing technology, coupled with China’s lithium resources being more abundant, so lithium-ion batteries have gradually become the mainstream development of power batteries and the first choice for new energy vehicles [11][12][13][16,17,18]. Lithium-ion batteries can be mainly categorized into lithium iron phosphate batteries and ternary batteries. In the early stage of the development of new energy vehicles, lithium iron phosphate batteries occupied a major market share. With the battery energy density included in the subsidy reference, ternary batteries were gradually promoted and used in new energy vehicles. But since 2020, the new energy vehicle subsidy policy has gradually sloped back, and the era of high energy density in exchange for high subsidies has quietly come to an end; lithium iron phosphate batteries, which are better in safety and more cost-effective, have re-entered the field of vision of most automobile enterprises; and the proportion of various types of power battery installations from 2018 to 2022 is shown in Figure 13.
Figure 13. Proportion of installed capacity of various types of power batteries from 2018 to 2022 (data from China Association of Automobile Manufacturers).
3. Recycling Methods of Power Battery
The lithium-ion battery recycling route is shown in Figure 24. Power batteries are installed in new energy vehicles after leaving the factory. With the increase in the number of times they are used, the capacity and attenuation performance of the battery gradually decreases. When its performance drops to 70%, it no longer meets the use of electric vehicles, but it can still be used for occasions with low requirements for the performance of the battery, such as energy storage systems, low-speed electric transportation, and at this time, it enters the stage of echelon utilization [15][16][20,21]. When the performance of the battery further declines to less than 40%, the value of the echelon utilization is also lost. At this time, the battery can be recycled through the dismantling and recycling methods. According to the above analysis, the method of recycling power batteries can be divided into echelon utilization and recycling.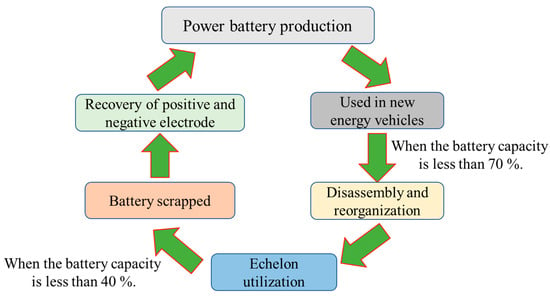
Figure 24.
The recycling route of lithium-ion batteries.
3.1. Echelon Utilization
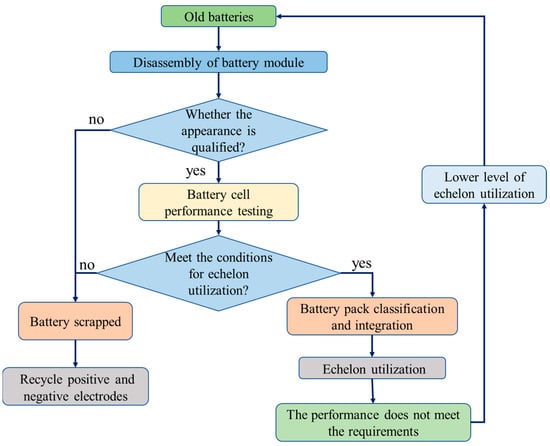
Generally, power batteries can be used as a power source for new energy vehicles for about 5 years, but their full life cycle from the beginning of use to the complete depletion of energy is about 20 years, which means that vehicle power batteries still have a residual life of about 15 years after scrapping. The echelon utilization is to use the end-of-life vehicle power batteries for other fields with lower requirements on battery performance, the process of which is shown in
Figure 3 [17] and usually includes steps such as battery pack disassembly, battery sieving, battery restructuring, and battery module system integration. The decommissioned power battery module needs to be tested twice after disassembly. For the first time, the appearance inspection is used to judge whether the appearance of the battery is qualified, and the battery with bulge, leakage, and deformation is eliminated. The second is the performance test to determine whether the basic performance of the battery meets the requirements and to eliminate the battery with a capacity lower than 40% of the original capacity, abnormal terminal voltage, or excessive internal resistance. The batteries that are qualified in the two tests are classified and integrated to achieve echelon utilization. The unqualified batteries can only be recycled via regenerative utilization.
5 [22] and usually includes steps such as battery pack disassembly, battery sieving, battery restructuring, and battery module system integration. The decommissioned power battery module needs to be tested twice after disassembly. For the first time, the appearance inspection is used to judge whether the appearance of the battery is qualified, and the battery with bulge, leakage, and deformation is eliminated. The second is the performance test to determine whether the basic performance of the battery meets the requirements and to eliminate the battery with a capacity lower than 40% of the original capacity, abnormal terminal voltage, or excessive internal resistance. The batteries that are qualified in the two tests are classified and integrated to achieve echelon utilization. The unqualified batteries can only be recycled via regenerative utilization.

Disassembling is the first step of power battery recycling, and the disassembling method and disassembling efficiency directly affect the echelon utilization. Wegener et al. [18] manually disassembled the hybrid powertrain of an Audi Q5 automobile, derived a disassembly sequence based on a prioritized relationship matrix, and illustrated the disassembly. Some scholars have attempted to introduce automated techniques to battery disassembly.
Disassembling is the first step of power battery recycling, and the disassembling method and disassembling efficiency directly affect the echelon utilization. Wegener et al. [23] manually disassembled the hybrid powertrain of an Audi Q5 automobile, derived a disassembly sequence based on a prioritized relationship matrix, and illustrated the disassembly. Some scholars have attempted to introduce automated techniques to battery disassembly.
The state of charge (SOC) and state of health (SOH) of the battery are key factors in determining the occasions for echelon utilization [19]. Therefore, after completing the battery pack disassembly, the disassembled battery cells need to be evaluated for SOC and SOH. SOC is a hidden state quantity, which is difficult to obtain directly from the sensor and can only be estimated based on the parameters of the external characteristics of the battery (terminal voltage, current, temperature). The traditional methods for estimating the SOC of a battery include the open-circuit voltage method [20][21], the internal resistance method [22][23], and the ampere-time integration method [24][25]. With the continuous development of related technologies, the estimation methods and accuracy of the SOC are constantly being revolutionized.
The state of charge (SOC) and state of health (SOH) of the battery are key factors in determining the occasions for echelon utilization [26]. Therefore, after completing the battery pack disassembly, the disassembled battery cells need to be evaluated for SOC and SOH. SOC is a hidden state quantity, which is difficult to obtain directly from the sensor and can only be estimated based on the parameters of the external characteristics of the battery (terminal voltage, current, temperature). The traditional methods for estimating the SOC of a battery include the open-circuit voltage method [27,28], the internal resistance method [29,30], and the ampere-time integration method [31,32]. With the continuous development of related technologies, the estimation methods and accuracy of the SOC are constantly being revolutionized. Estimating the SOC by modeling is a hot topic in current research.
The estimation methods of SOH state can be divided into two kinds: offline estimation method and online estimation method. Among them, offline estimation has the advantages of simplicity and low computation, but it demands strict requirements on the test environment, and the experiments are too time-consuming. In recent years, researchers have proposed some online estimation methods based on offline estimation, such as the slip film observer method [26], voltage curve fitting method [27][28], neural network method [29], and fuzzy logic inference method [30][31], which can realize real-time online estimation of SOH. Nevertheless, SOH evaluation is still difficult due to the many factors affecting the SOH state, including ambient temperature, charge/discharge multiplier, depth of discharge, and charge/discharge cycle, and SOH is a highly nonlinear time-varying system [32].
The estimation methods of SOH state can be divided into two kinds: offline estimation method and online estimation method. Among them, offline estimation has the advantages of simplicity and low computation, but it demands strict requirements on the test environment, and the experiments are too time-consuming. In recent years, researchers have proposed some online estimation methods based on offline estimation, such as the slip film observer method [37], voltage curve fitting method [38,39], neural network method [40], and fuzzy logic inference method [41,42], which can realize real-time online estimation of SOH. Nevertheless, SOH evaluation is still difficult due to the many factors affecting the SOH state, including ambient temperature, charge/discharge multiplier, depth of discharge, and charge/discharge cycle, and SOH is a highly nonlinear time-varying system [43].
3.2. Regenerative Utilization
Another resource utilization path for decommissioned power batteries is regeneration, where the batteries that cannot be utilized in the echelon are scrapped, and the valuable metals are refined for the production of new power batteries. Among them, the positive electrode of power batteries contains abundant lithium, cobalt, nickel, manganese, and other metal elements, which have high recycling value and are the core of recycling and reutilization of decommissioned power batteries [33]. Scholars have conducted a lot of research on the regeneration of decommissioned power batteries and summarized the main processes, which mainly include pretreatment, separation and extraction, and product preparation, as shown in
Another resource utilization path for decommissioned power batteries is regeneration, where the batteries that cannot be utilized in the echelon are scrapped, and the valuable metals are refined for the production of new power batteries. Among them, the positive electrode of power batteries contains abundant lithium, cobalt, nickel, manganese, and other metal elements, which have high recycling value and are the core of recycling and reutilization of decommissioned power batteries [44]. Scholars have conducted a lot of research on the regeneration of decommissioned power batteries and summarized the main processes, which mainly include pretreatment, separation and extraction, and product preparation, as shown in
Figure 4.
6.
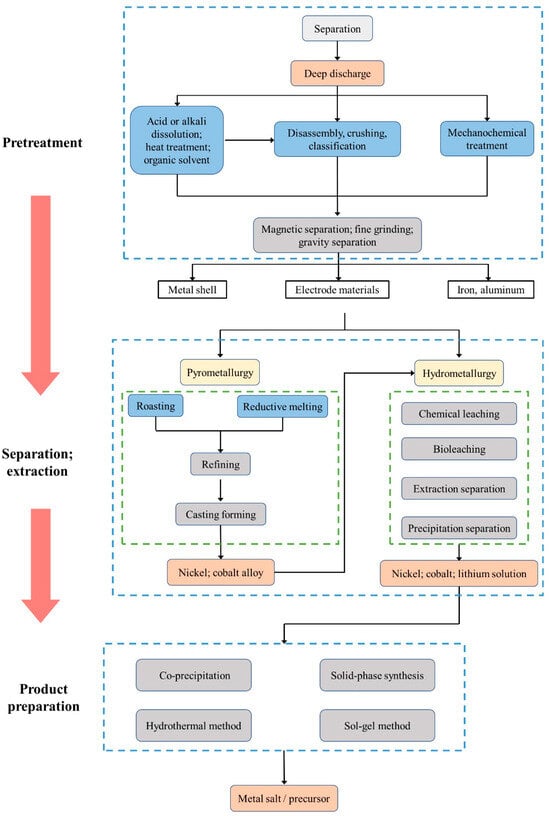

Figure 46. Recycling process of decommissioned power battery [34][6].
3.2.1. Pretreatment Process
The purpose of pretreatment is to effectively separate the components in the battery and enrich the valuable metal elements, which mainly includes deep discharge, disassembly, classification, crushing and screening, separation, and mechanochemical treatment.
Lithium-ion batteries have a high energy density. At the start of recycling, the batteries still have residual power, so the first step in pre-treatment is to deeply discharge the batteries to avoid spontaneous combustion or explosion during the pre-treatment process [35]. Common discharge methods include immersion, resistance, and perforated discharge.
Lithium-ion batteries have a high energy density. At the start of recycling, the batteries still have residual power, so the first step in pre-treatment is to deeply discharge the batteries to avoid spontaneous combustion or explosion during the pre-treatment process [45]. Common discharge methods include immersion, resistance, and perforated discharge.
Disassembly and classification are the key link to realize the regeneration of decommissioned power batteries. Initially, the disassembly and classification were manually realized, and the disassembly efficiency was low. With the development of mechanical and automation technology, researchers have invented numerous devices and systems for the automatic disassembly of power batteries [36].
Disassembly and classification are the key link to realize the regeneration of decommissioned power batteries. Initially, the disassembly and classification were manually realized, and the disassembly efficiency was low. With the development of mechanical and automation technology, researchers have invented numerous devices and systems for the automatic disassembly of power batteries [49].
The disassembled and classified power batteries are crushed; then, physical sorting methods (re-election, electrical, and magnetic separation) can be used to realize the further separation and recovery of different component materials. The crushing process produces exhaust gases, liquids, and residues that are hazardous to human health [37]. Scholars have attempted to improve treatment to avoid the release of toxic and harmful gases.
The disassembled and classified power batteries are crushed; then, physical sorting methods (re-election, electrical, and magnetic separation) can be used to realize the further separation and recovery of different component materials. The crushing process produces exhaust gases, liquids, and residues that are hazardous to human health [50]. Scholars have attempted to improve treatment to avoid the release of toxic and harmful gases.
Crushing and sieving are difficult to dislodge the positive and negative electrode materials from the aluminum and copper foils, so further separation and extraction is required, and the main methods are alkaline dissolution [38], heat treatment [39], organic solvent [40] and ultrasonic treatment [41]. The alkaline dissolution method takes advantage of that the positive electrode material can be stabilized in alkaline conditions, and the aluminum foil will be completely dissolved to make the positive electrode material off. The method is more reported and widely used, so the study will not be introduced in detail. The small safety factor is the main drawback of this method, as the operation requires a high concentration of alkaline solution. The heat treatment method uses high temperatures to volatilize or pyrolyze the binder to separate the positive material from the aluminum foil.
Crushing and sieving are difficult to dislodge the positive and negative electrode materials from the aluminum and copper foils, so further separation and extraction is required, and the main methods are alkaline dissolution [56], heat treatment [57], organic solvent [58] and ultrasonic treatment [59]. The alkaline dissolution method takes advantage of that the positive electrode material can be stabilized in alkaline conditions, and the aluminum foil will be completely dissolved to make the positive electrode material off. The method is more reported and widely used, so the study will not be introduced in detail. The small safety factor is the main drawback of this method, as the operation requires a high concentration of alkaline solution. The heat treatment method uses high temperatures to volatilize or pyrolyze the binder to separate the positive material from the aluminum foil.
3.2.2. Separation and Extraction
The pretreatment process realizes the enrichment of valuable metal components, but further recovery of valuable metals such as cobalt, lithium, and nickel requires further separation and extraction of the pretreated products. Pyrometallurgy and hydrometallurgy are the most common separation and purification methods at present.- (1)
-
PyrometallurgyPyrometallurgy refers to the removal of the organic binder in the electrode material with the help of high temperature, and at the same time, the metal and its compounds therein undergo a series of chemical reactions, and finally, the extraction of the target metal material is realized through subsequent separation [65]. The pyrometallurgical process is widely studied because of its simple operation, good adaptability of raw materials, and easy realization of large-scale production.
- (2)
-
Hydrometallurgy
3.2.3. Product Preparation
The products of pyrometallurgy can be used to produce cobalt-nickel alloys through refining and casting. The products of hydrometallurgy can be used to prepare single-metal salts and to resynthesize battery materials. When preparing single metal salts, nickel, cobalt, and lithium salts need to be separated from the leach solution and further prepared into cobalt sulfate, cobalt chloride, nickel sulfate, nickel carbonate, lithium carbonate, and other products. Resynthesizing battery materials refers to the preparation of positive material precursors by adjusting the pH value and the ratio of metal components after removing the impurity metals from the leach solution, including co-precipitation [43][44][84,85], sol-gel [45][86], high-temperature solid-phase synthesis [46][87], and hydrothermal methods [47][88].3.3. Direct Regeneration of Positive Electrode Materials
The direct regeneration of positive electrode materials improves the electrochemical performance by adding lithium to the decommissioned positive electrode materials without destroying their crystal structure [48][49][89,90]. Compared with other recycling technologies, direct regeneration has the outstanding advantages of a simple process, low cost, and environmental friendliness, and the regenerated positive electrode material can be reused, so it has been developed rapidly. Lithium depletion is the main reason for the failure of lithium-ion battery’s positive electrode materials. The purpose of direct regeneration technology is to combine different lithium replenishment technologies with heat treatment, thus realizing the direct repair of used batteries, including direct solid-state calcination, hydrothermal regeneration, molten salt lithiation, and electrochemical lithium replenishment. Figure 58 shows the direct regeneration of waste ternary materials via lithium supplementation.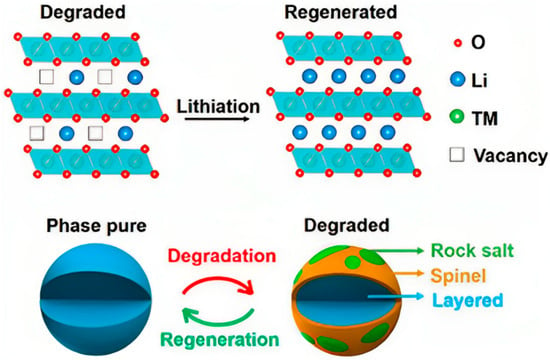 Direct regeneration of waste ternary materials via lithium supplementation [91].Direct solid-state calcination is the most widely used direct regeneration method, especially for batteries with single-component positive electrode materials such as LiCoO2, LiFePO4, and NCM. Chi et al. [51][92] directly utilized Li2CO3 present on the surface of the waste positive electrode material as a lithium source, placed it together with the positive electrode material to be regenerated in an oxygen atmosphere, and calcined them for 12 h at a temperature of 850 °C to achieve the regeneration purpose.The hydrothermal regeneration method is to uniformly distribute the lithium source in the aqueous solution so that it is in direct contact with the positive electrode material, thereby supplementing lithium for the failed positive electrode material.
Direct regeneration of waste ternary materials via lithium supplementation [91].Direct solid-state calcination is the most widely used direct regeneration method, especially for batteries with single-component positive electrode materials such as LiCoO2, LiFePO4, and NCM. Chi et al. [51][92] directly utilized Li2CO3 present on the surface of the waste positive electrode material as a lithium source, placed it together with the positive electrode material to be regenerated in an oxygen atmosphere, and calcined them for 12 h at a temperature of 850 °C to achieve the regeneration purpose.The hydrothermal regeneration method is to uniformly distribute the lithium source in the aqueous solution so that it is in direct contact with the positive electrode material, thereby supplementing lithium for the failed positive electrode material.4. Conclusions
The echelon utilization is an important method for the resource utilization of decommissioned power batteries. Restricted by technology and cost, it is difficult to accurately assess the SOH and SOC of the battery. It is the main development direction of the echelon utilization to improve the manufacturing technology of the power battery and reduce the cost of pre-processing. In addition, the development of new methods for rapid and safe assessment of SOH and SOC is also the focus of future researchers.Regenerative utilization is the main method of resource utilization of decommissioned power batteries. Pre-treatment is the key to the regenerative utilization of power batteries, in which safety, fast, deep discharge, and efficient separation of battery components are the main challenges, and the combination of multiple separation methods is an inevitable choice for the battery pre-treatment link. Pyrometallurgy and hydrometallurgy are the main methods of separation and extraction. Compared with pyrometallurgy, hydrometallurgy has lower energy consumption and less pollution, but its high cost limits its large-scale industrial application. Seeking cheap and efficient leaching agents is the focus of future research.The direct regeneration of positive electrode materials is the most promising recycling method for decommissioned power batteries. Compositional diversity and uncertainty in the degree of failure are the main factors hindering the direct regeneration of positive electrode materials. Developing suitable pretreatment processes to ensure the extraction of higher purity positive electrode materials without destroying the original crystal structure, as well as exploring how to accurately and rapidly determine the lack of lithium in the waste positive electrode materials, are the future directions for the direct regeneration of positive electrode materials.
