
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Giuseppe De Giorgio | -- | 1357 | 2024-02-19 12:26:25 | | | |
| 2 | Giuseppe De Giorgio | + 2 word(s) | 1359 | 2024-02-19 12:33:29 | | | | |
| 3 | Camila Xu | Meta information modification | 1359 | 2024-02-28 09:48:26 | | | | |
| 4 | Giuseppe De Giorgio | + 1 word(s) | 1360 | 2024-03-20 15:43:03 | | |
Video Upload Options
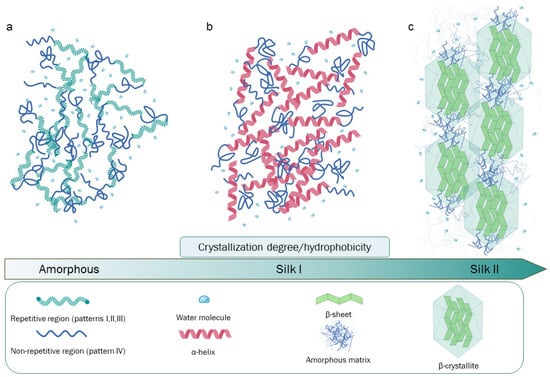
Silk fibroin is the principal component of raw silk and represents an extensively studied and utilized biopolymer. Silk fibroin is composed by three chains, light, heavy, and P25 protein. Heavy chain is rigorously organized in redundant aminoacidic sequences rich in glycine and alanine, secondary structure is organized in anti-parallel β-sheets that in turn form β-crystallites stacked in nano-fibrils. Those peculiar fibroin’s structural and compositional elements are crucial to determine the excellent physical properties, such as strength and toughness. Besides these characteristics, the processability and biocompatibility have attracted significant attention for the fabrication of several biomaterials suitable in many fields of application.
1. Introduction
2. The Composition and Structure of Silk Fibroin
References
- Bucciarelli, A.; Motta, A. Use of Bombyx Mori Silk Fibroin in Tissue Engineering: From Cocoons to Medical Devices, Challenges, and Future Perspectives. Biomater. Adv. 2022, 139, 212982.
- Craig, C.L. Evolution of arthropod silks. Annu. Rev. Èntomol. 1997, 42, 231–267.
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A Comprehensive Review of Recent Advances in Silk Sericin: Extraction Approaches, Structure, Biochemical Characterization, and Biomedical Applications. Int. J. Biol. Macromol. 2023, 250, 126067.
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect Silk: One Name, Many Materials. Annu. Rev. Entomol. 2010, 55, 171–188.
- Kwak, H.W.; Ju, J.E.; Shin, M.; Holland, C.; Lee, K.H. Sericin Promotes Fibroin Silk i Stabilization Across a Phase-Separation. Biomacromolecules 2017, 18, 2343–2349.
- Michaille, J.J.; Garel, A.; Prudhomme, J.C. The expression of five middle silk gland specific genes is territorially regulated during the larval development of Bombyx mori. Insect Biochem. 1989, 19, 19–27.
- Mondal, M.; Trivedy, K.; Nirmal Kumar, S. The Silk Proteins, Sericin and Fibroin in Silkworm, Bombyx Mori Linn—A Review. Casp. J. Environ. Sci. 2007, 5, 63–76.
- Chen, F.; Porter, D.; Vollrath, F. Structure and Physical Properties of Silkworm Cocoons. J. R. Soc. Interface 2012, 9, 2299–2308.
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 1–19.
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the Structure of Bombyx Mori Silk Fibroin by NMR. Macromolecules 2015, 48, 2345–2357.
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx Mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-Chain, L-Chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528.
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276.
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombyx mori. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1999, 1432, 92–103.
- Zhou, C.-Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine Organization of Bombyx Mori Fibroin Heavy Chain Gene. Nucleic Acids Res. 2000, 28, 2413–2419.
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of PH and Charge on Silk Protein Assembly in Insects and Spiders. Appl. Phys. A Mater. Sci. Process 2006, 82, 223–233.
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk Fibroin: Structural Implications of a Remarkable Amino Acid Sequence. Proteins: Struct. Funct. Genet. 2001, 44, 119–122.
- Lotz, B.; Cesari, F.C. The Chemical Structure and the crystalline structures of Bombyx Mori Silk Fibroin. Biochimie 1979, 61, 205–214.
- Marsh, R.E.; Corey, R.B.; Pauling, L. An Investigation of the Structure of Silk Fibroin. Biochimica Biophysica Acta 1955, 16, 1–34.
- Yamaguchi’, K.; Kikuchi’, Y.; Takagi’, T.; Kikuchi’, A.; Oyama’, F.; Shimural, K.; Mizuno’s, S. Primary Structure of the Silk Fibroin Light Chain Determined by CDNA Sequencing and Peptide Analysis. J. Mol. Biol. 1989, 210, 127–139.
- Asakura, T.; Ohgo, K.; Komatsu, K.; Kanenari, M.; Okuyama, K. Refinement of Repeated β-Turn Structure for Silk I Conformation of Bombyx Mori Silk Fibroin Using13C Solid-State NMR and X-Ray Diffraction Methods. Macromolecules 2005, 38, 7397–7403.
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural Origin of the Strain-Hardening of Spider Silk. Adv. Funct. Mater. 2011, 21, 772–778.
- Nguyen, A.T.; Huang, Q.L.; Yang, Z.; Lin, N.; Xu, G.; Liu, X.Y. Crystal Networks in Silk Fibrous Materials: From Hierarchical Structure to Ultra Performance. Small 2014, 11, 1039–1054.
- Wilson, D.; Valluzzi, R.; Kaplan, D. Conformational Transitions Model Silk Peptides. Biophys. J. 2000, 78, 2690–2701.
- Matsumoto, A.; Lindsay, A.; Abedian, B.; Kaplan, D.L. Silk Fibroin Solution Properties Related to Assembly and Structure. Macromol. Biosci. 2008, 8, 1006–1018.
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II Studied by Fast Scanning Calorimetry. Acta Biomater. 2017, 55, 323–332.
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of silk III at the air-water interface. Int. J. Biol. Macromol. 1999, 24, 237–242.




