Silk fibroin is the principal component of raw silk and represents an extensively studied and utilized biopolymer. Silk fibroin is composed by three chains, light, heavy, and P25 protein. Heavy chain is rigorously organized in redundant aminoacidic sequences rich in glycine and alanine, secondary structure is organized in anti-parallel β-sheets that in turn form β-crystallites stacked in nano-fibrils. Those peculiar fibroin’s structural and compositional elements are crucial to determine the excellent physical properties, such as strength and toughness. Besides these characteristics, the processability and biocompatibility have attracted significant attention for the fabrication of several biomaterials suitable in many fields of application.
- silk
- fibroin
- tissue engineering
- regenerative medicine
- biomedicine
- bone regeneration
1. Introduction
2. The Composition and Structure of Silk Fibroin
The composition of raw silkworm silk is as follows: approximately 70–80% fibroin, 20–30% sericin (SR), and a small percentage of other components such as wax, inorganic matter, and pigments [5]. SR encompasses a family of glycoproteins generated by alternative splicing events that take place in the larvae-secreting organs (silk glands). The aminoacidic composition of these glycoproteins is characterized by a high serin abundance [6]. SR’s serin-rich domains allow for interaction with water molecules, making SR sticky and imbuing it with a glue-like consistency from a macroscopic/mechanical point of view. Due to these peculiar properties, during the cocooning process, two fibroin filaments are naturally “glued” together with SR, protecting the complex from biological and atmospheric agents [7][8][9]. Silk fribroin (SF) belongs to a distinct class of glycoproteins, and its structure is characterized by a heavy chain (Hc) with an approximate molecular weight (MW) of 350 kDa and a light chain (Lc) of about 25 kDa, which are linked together by a disulfide bond, forming the (H-L) complex. This complex, in turn, interacts with a glycosylated protein of 27 kDa, named P25, with hydrophobic interactions in a 6:6:1 molar ratio, forming an elementary unit that is crucial for SF secretion, the spinning process, and fiber formation. In particular, during the secretion process, Lc and P25 exert structural control to SF, playing a protective role in the silk glands [10][11]. Lc has a non-repetitive and amorphous structure that is more hydrophilic than Hc. For this reason, during the secretion step, Lc protects against excessive crystallization, while Hc prevents premature SF denaturation. During secretion, a further contribution is given by P25, whose biological role is to maintain the stability of the H-L complex. An additional role for the P25 protein has also been proposed. Specifically, it may induce molecular chaperone activity, assisting the correct folding state of SF. P25 is less stable than the H-L complex, and it degrades after the SF fiber formation process [12][13]. The primary structure of Hc is the key for the structural and biological roles of SF, being characterized by a complex aminoacidic composition and patterning. The four most represented amino acids are as follows: glycine (Gly, 46%), alanine (Ala, 30%), serine (Ser, 12%), and tyrosine (Tyr, 5%) [14]. The highly repetitive patterns present in Hc can be classified into four principal typologies: (I) GAGAGS, a pattern playing a critical role as a core component of the SF crystalline region; (II) GAGAGVGY–GAGAGY–GAGAGV, which are three sequences rich in aromatic and hydrophobic residues, principally located in the semicrystalline region of the protein; (III) this pattern is highly similar to (I) but has the presence of an “AAS” β-sheet breaking pattern located at the c-terminal; (IV) these hydrophilic, non-repetitive patterns, which are also called linkers, lack a high-ordered structure, and hence, they may be found in combination with crystallized regions in SF. Patterns such as (IV), in particular, have different aminoacidic compositions and are interspersed through the (I, II, III) patterns [14][15][16]. Globally, 12 (I, II, or III) repetitive patterns interspaced by 11 (IV) non-repetitive amorphous regions are present in the primary structure of Hc (Figure 1).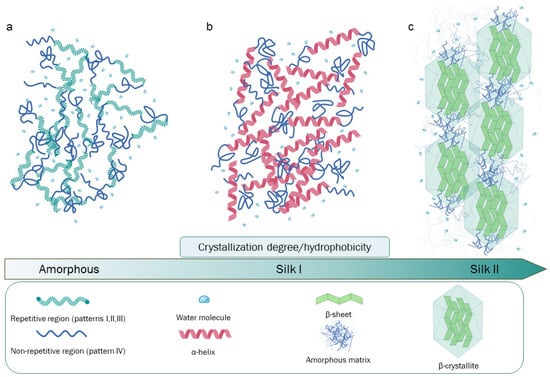
References
- Bucciarelli, A.; Motta, A. Use of Bombyx Mori Silk Fibroin in Tissue Engineering: From Cocoons to Medical Devices, Challenges, and Future Perspectives. Biomater. Adv. 2022, 139, 212982.
- Craig, C.L. Evolution of arthropod silks. Annu. Rev. Èntomol. 1997, 42, 231–267.
- Saad, M.; El-Samad, L.M.; Gomaa, R.A.; Augustyniak, M.; Hassan, M.A. A Comprehensive Review of Recent Advances in Silk Sericin: Extraction Approaches, Structure, Biochemical Characterization, and Biomedical Applications. Int. J. Biol. Macromol. 2023, 250, 126067.
- Sutherland, T.D.; Young, J.H.; Weisman, S.; Hayashi, C.Y.; Merritt, D.J. Insect Silk: One Name, Many Materials. Annu. Rev. Entomol. 2010, 55, 171–188.
- Kwak, H.W.; Ju, J.E.; Shin, M.; Holland, C.; Lee, K.H. Sericin Promotes Fibroin Silk i Stabilization Across a Phase-Separation. Biomacromolecules 2017, 18, 2343–2349.
- Michaille, J.J.; Garel, A.; Prudhomme, J.C. The expression of five middle silk gland specific genes is territorially regulated during the larval development of Bombyx mori. Insect Biochem. 1989, 19, 19–27.
- Mondal, M.; Trivedy, K.; Nirmal Kumar, S. The Silk Proteins, Sericin and Fibroin in Silkworm, Bombyx Mori Linn—A Review. Casp. J. Environ. Sci. 2007, 5, 63–76.
- Chen, F.; Porter, D.; Vollrath, F. Structure and Physical Properties of Silkworm Cocoons. J. R. Soc. Interface 2012, 9, 2299–2308.
- Kunz, R.I.; Brancalhão, R.M.C.; Ribeiro, L.D.F.C.; Natali, M.R.M. Silkworm Sericin: Properties and Biomedical Applications. BioMed Res. Int. 2016, 2016, 1–19.
- Asakura, T.; Okushita, K.; Williamson, M.P. Analysis of the Structure of Bombyx Mori Silk Fibroin by NMR. Macromolecules 2015, 48, 2345–2357.
- Inoue, S.; Tanaka, K.; Arisaka, F.; Kimura, S.; Ohtomo, K.; Mizuno, S. Silk Fibroin of Bombyx Mori Is Secreted, Assembling a High Molecular Mass Elementary Unit Consisting of H-Chain, L-Chain, and P25, with a 6:6:1 Molar Ratio. J. Biol. Chem. 2000, 275, 40517–40528.
- Tanaka, K.; Inoue, S.; Mizuno, S. Hydrophobic interaction of P25, containing Asn-linked oligosaccharide chains, with the H-L complex of silk fibroin produced by Bombyx mori. Insect Biochem. Mol. Biol. 1999, 29, 269–276.
- Tanaka, K.; Kajiyama, N.; Ishikura, K.; Waga, S.; Kikuchi, A.; Ohtomo, K.; Takagi, T.; Mizuno, S. Determination of the site of disulfide linkage between heavy and light chains of silk fibroin produced by Bombyx mori. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzym. 1999, 1432, 92–103.
- Zhou, C.-Z.; Confalonieri, F.; Medina, N.; Zivanovic, Y.; Esnault, C.; Yang, T.; Jacquet, M.; Janin, J.; Duguet, M.; Perasso, R.; et al. Fine Organization of Bombyx Mori Fibroin Heavy Chain Gene. Nucleic Acids Res. 2000, 28, 2413–2419.
- Foo, C.W.P.; Bini, E.; Hensman, J.; Knight, D.P.; Lewis, R.V.; Kaplan, D.L. Role of PH and Charge on Silk Protein Assembly in Insects and Spiders. Appl. Phys. A Mater. Sci. Process 2006, 82, 223–233.
- Zhou, C.Z.; Confalonieri, F.; Jacquet, M.; Perasso, R.; Li, Z.G.; Janin, J. Silk Fibroin: Structural Implications of a Remarkable Amino Acid Sequence. Proteins: Struct. Funct. Genet. 2001, 44, 119–122.
- Lotz, B.; Cesari, F.C. The Chemical Structure and the crystalline structures of Bombyx Mori Silk Fibroin. Biochimie 1979, 61, 205–214.
- Marsh, R.E.; Corey, R.B.; Pauling, L. An Investigation of the Structure of Silk Fibroin. Biochimica Biophysica Acta 1955, 16, 1–34.
- Yamaguchi’, K.; Kikuchi’, Y.; Takagi’, T.; Kikuchi’, A.; Oyama’, F.; Shimural, K.; Mizuno’s, S. Primary Structure of the Silk Fibroin Light Chain Determined by CDNA Sequencing and Peptide Analysis. J. Mol. Biol. 1989, 210, 127–139.
- Asakura, T.; Ohgo, K.; Komatsu, K.; Kanenari, M.; Okuyama, K. Refinement of Repeated β-Turn Structure for Silk I Conformation of Bombyx Mori Silk Fibroin Using13C Solid-State NMR and X-Ray Diffraction Methods. Macromolecules 2005, 38, 7397–7403.
- Du, N.; Yang, Z.; Liu, X.Y.; Li, Y.; Xu, H.Y. Structural Origin of the Strain-Hardening of Spider Silk. Adv. Funct. Mater. 2011, 21, 772–778.
- Nguyen, A.T.; Huang, Q.L.; Yang, Z.; Lin, N.; Xu, G.; Liu, X.Y. Crystal Networks in Silk Fibrous Materials: From Hierarchical Structure to Ultra Performance. Small 2014, 11, 1039–1054.
- Wilson, D.; Valluzzi, R.; Kaplan, D. Conformational Transitions Model Silk Peptides. Biophys. J. 2000, 78, 2690–2701.
- Matsumoto, A.; Lindsay, A.; Abedian, B.; Kaplan, D.L. Silk Fibroin Solution Properties Related to Assembly and Structure. Macromol. Biosci. 2008, 8, 1006–1018.
- Cebe, P.; Partlow, B.P.; Kaplan, D.L.; Wurm, A.; Zhuravlev, E.; Schick, C. Silk I and Silk II Studied by Fast Scanning Calorimetry. Acta Biomater. 2017, 55, 323–332.
- Valluzzi, R.; Gido, S.P.; Muller, W.; Kaplan, D.L. Orientation of silk III at the air-water interface. Int. J. Biol. Macromol. 1999, 24, 237–242.
