
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Joji M. Otaki | -- | 3835 | 2024-02-19 10:14:06 | | | |
| 2 | Lindsay Dong | Meta information modification | 3835 | 2024-02-20 01:44:00 | | |
Video Upload Options
Compositional changes in soil microbes associated with decreases in abundance and species diversity were reported, especially in heavily contaminated areas of both Chernobyl and Fukushima, which may accompany explosions of radioresistant species. In Chernobyl, the population size of soil microbes remained low for at least 20 years after the accident, and the abundance of plant-associated microbes, which are related to the growth and defense systems of plants, possibly decreased. These reported changes in microbes likely affect soil conditions and alter plant physiology. These microbe-mediated effects may then indirectly affect insect herbivores through food-mass-mediated, pollen-mediated, and metabolite-mediated interactions. Metabolite-mediated interactions may be a major pathway for ecological impacts at low pollution levels and could explain the decreases in insect herbivores in Fukushima.
1. Introduction
2. Multiple Pathways for Biological Effects
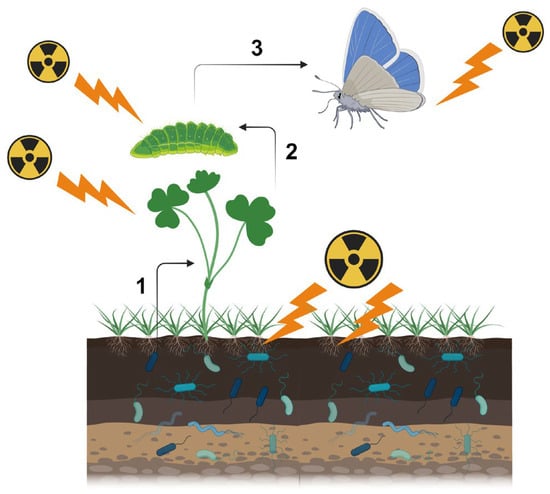
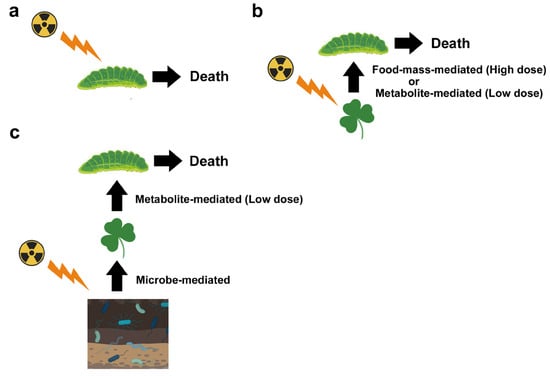
3. Soil Microbes and Soil Invertebrates
3.1. Chernobyl Studies
3.2. Fukushima Studies
3.3. Commonalities between Chernobyl and Fukushima
4. Plant-Associated Microbes
4.1. Chernobyl Studies
4.2. Fukushima Studies
4.3. Commonalities between Chernobyl and Fukushima
5. Plants and Insect Herbivores
5.1. Food-Mass-Mediated Indirect Effects
5.2. Pollen-Mediated Indirect Effects
5.3. Metabolite-Mediated Indirect Effect
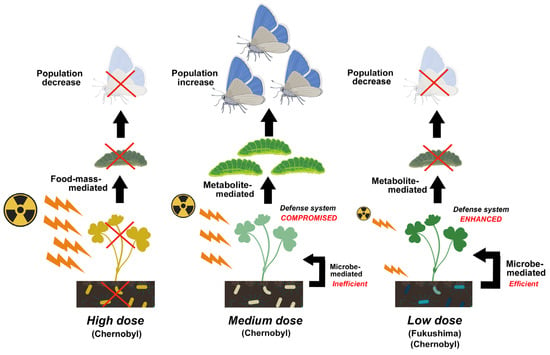
6. Conclusions
References
- Wagner, H.N., Jr. Atoms for peace (and health). J. Nucl. Med. 2004, 45, 24N.
- Babic, R.R.; Babic, G.S.; Babic, S.R.; Babic, N.R. 120 years since the discovery of x-rays. Med. Pregl. 2016, 69, 323–330.
- Nüsslin, F. Wilhelm Conrad Röntgen: The scientist and his discovery. Phys. Med. 2020, 79, 65–68.
- Brodsky, A.; Kathren, R.L.; Willis, C.A. History of the medical uses of radiation: Regulatory and voluntary standards of protection. Health Phys. 1995, 69, 783–823.
- Reed, A.B. History of radiation use in medicine. J. Vasc. Surg. 2011, 53 (Suppl. S1), 3S–5S.
- Scatliff, J.H.; Morris, P.J. From Röntgen to magnetic resonance imaging: The history of medical imaging. N. C. Med. J. 2014, 75, 111–113.
- Ma, L.; Kong, F.; Sun, K.; Wang, T.; Guo, T. From classical radiation to modern radiation: Past, present, and future of radiation mutation breeding. Front. Public Health 2021, 9, 768071.
- Riviello-Flores, M.d.l.L.; Cadena-Iñiguez, J.; Ruiz-Posadas, L.d.M.; Arévalo-Galarza, M.d.L.; Castillo-Juárez, I.; Hernández, M.S.; Castillo-Martínez, C.R. Use of gamma radiation for the genetic improvement of underutilized plant varieties. Plants 2022, 11, 1161.
- Whitmore, G.F. One hundred years of X rays in biological research. Radiat. Res. 1995, 144, 148–159.
- Geras’kin, S.A.; Fesenko, S.V.; Alexakhin, R.M. Effects of non-human species irradiation after the Chernobyl NPP accident. Environ. Int. 2008, 34, 880–897.
- Yoshida, N.; Kanda, J. Geochemistry. Tracking the Fukushima radionuclides. Science 2012, 336, 1115–1116.
- International Atomic Energy Agency (IAEA). Environmental Consequences of the Chernobyl Accident and their Remediation: Twenty Years of Experience; Radiological Assessment Reports Series No. 8; IAEA: Vienna, Austria, 2006.
- Aliyu, A.S.; Evangeliou, N.; Mousseau, T.A.; Wu, J.; Ramli, A.T. An overview of current knowledge concerning the health and environmental consequences of the Fukushima Daiichi Nuclear Power Plant (FDNPP) accident. Environ. Int. 2015, 85, 213–228.
- Ministry of the Environment (Government of Japan). Chapter 4. Concept of radiological protection. In BOOKLET to Provide Basic Information Regarding Health Effects of Radiation, 3rd ed.; Ministry of the Environment (Government of Japan): Tokyo, Japan, 2016; Available online: https://www.env.go.jp/en/chemi/rhm/basic-info/1st/04.html (accessed on 13 January 2024).
- International Atomic Energy Agency (IAEA). Effects of Ionizing Radiation on Plants and Animals at Levels Implied by Current Radiation Protection Standards; Technical Reports Series No. 332; IAEA: Vienna, Austria, 1992.
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Sources and Effects of Ionizing Radiation UNSCEAR 1996 Report to the General Assembly, with Scientific Annex; United Nations: New York, NY, USA, 1996.
- European Commission. Protection of the Environment; European Union: Luxembourg, 2014; Available online: https://energy.ec.europa.eu/system/files/2014-11/177_0.pdf (accessed on 13 January 2024).
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 168.
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21.
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; InTechOpen: London, UK, 2018; pp. 49–71.
- Otaki, J.M.; Sakauchi, K.; Taira, W. The second decade of the blue butterfly in Fukushima: Untangling the ecological field effects after the Fukushima nuclear accident. Integr. Environ. Assess. Manag. 2022, 18, 1539–1550.
- Beaugelin-Seiller, K.; Della-Vedova, C.; Garnier-Laplace, J. Is non-human species radiosensitivity in the lab a good indicator of that in the field? Making the comparison more robust. J. Environ. Radioact. 2020, 211, 105870.
- Woodwell, G.M.; Sparrow, A.H. Predicted and observed effects of chronic gamma radiation on a near-climax forest ecosystem. Radiat. Botany 1963, 3, 231–237.
- Caffrey, E.; Leonard, M.; Napier, J.; Neville, D.; Higley, K. Radioecology: Why Bother? J. Environ. Prot. 2014, 5, 181–192.
- Bradshaw, C.; Kapustka, L.; Barnthouse, L.; Brown, J.; Ciffroy, P.; Forbes, V.; Geras’kin, S.; Kautsky, U.; Bréchignac., F. Using an Ecosystem Approach to complement protection schemes based on organism-level endpoints. J. Environ. Radioact. 2014, 136, 98–104.
- Brèchignac, F.; Doi, M. Challenging the current strategy of radiological protection of the environment: Arguments for an ecosystem approach. J. Environ. Radioact. 2009, 100, 1125–1134.
- Woodwell, G.M. Ecological Effects of Nuclear War; Brookhaven National Laboratory: Upton, NY, USA, 1963.
- Bréchignac, F.; Alexakhin, R.; Godoy, J.M.; Oughton, D.; Sheppard, S.; Strand, P. Integrating environment protection, a new challenge: Strategy of the International Union of Radioecology. Radioprotection 2008, 43, 339–356.
- Dalkvist, T.; Topping, C.J.; Forbes, V.E. Population-level impacts of pesticide-induced chronic effects on individuals depend more on ecology than toxicology. Ecotoxicol. Environ. Saf. 2009, 72, 1663–1672.
- Clements, W.H.; Rohr, J.R. Community responses to contaminants: Using basic ecological principles to predict ecotoxicological effects. Environ. Toxicol. Chem. 2009, 28, 1789–1800.
- Mothersill, C.; Seymour, C. Communication of ionising radiation signals—A tale of two fish. Int. J. Radiat. Biol. 2009, 85, 909–919.
- Møller, A.P.; Nishiumi, I.; Suzuki, H.; Ueda, K.; Mousseau, T.A. Differences in effects of radiation on abundance of animals in Fukushima and Chernobyl. Ecol. Indic. 2013, 24, 75–81.
- Møller, A.P.; Mousseau, T.A. Reduced abundance of insects and spiders linked to radiation at Chernobyl 20 years after the accident. Biol. Lett. 2009, 5, 356–359.
- Møller, A.P.; Mousseau, T.A. Efficiency of bio-indicators for low-level radiation under field conditions. Ecol. Indic. 2011, 11, 424–430.
- Turner, F.B. Effects of continuous irradiation on animal populations. Adv. Radiat. Biol. 1975, 5, 83–144.
- Lecomte-Pradines, C.; Bonzom, J.M.; Della-Vedova, C.; Beaugelin-Seiller, K.; Villenave, C.; Gaschak, S.; Coppin, F.; Dubourg, N.; Maksimenko, A.; Adam-Guillermin, C.; et al. Soil nematode assemblages as bioindicators of radiation impact in the Chernobyl Exclusion Zone. Sci. Total Environ. 2014, 490, 161–170.
- Møller, A.P.; Mousseau, T.A. Species richness and abundance of forest birds in relation to radiation at Chernobyl. Biol. Lett. 2007, 3, 483–486.
- Zhdanova, N.N.; Vasilevskaia, A.I.; Artyshkova, L.V.; Gavriliuk, V.I.; Lashko, T.N.; Sadovnikov, I.S. Kompleksy pochvennykh mikrokitsetov v zone vliianiia Chernobyl’skoĭ AES . Mikrobiol. Zh. 1991, 53, 3–9. (In Russian)
- Yablokov, A.V.; Nesterenko, V.B.; Nesterenko, A.V. Consequences of the Chernobyl catastrophe for public health and the environment 23 years later. Ann. N. Y. Acad. Sci. 2009, 1181, 318–326.
- Cordero, R.J.; Casadevall, A. Functions of fungal melanin beyond virulence. Fungal Biol. Rev. 2017, 31, 99–112.
- Dadachova, E.; Bryan, R.A.; Huang, X.; Moadel, T.; Schweitzer, A.D.; Aisen, P.; Nosanchuk, J.D.; Casadevall, A. Ionizing radiation changes the electronic properties of melanin and enhances the growth of melanized fungi. PLoS ONE 2007, 2, e457.
- Dadachova, E.; Casadevall, A. Ionizing radiation: How fungi cope, adapt, and exploit with the help of melanin. Curr. Opin. Microbiol. 2008, 11, 525–531.
- Romanovskaia, V.A.; Stoliar, S.M.; Malashenko, I.R.; Shatokhina, E.S. Vliianie radiatsii dlitel’nogo deĭstviia na raznoobrazie geterotrofnykh bakteriĭ v pochvakh 10-kilometrovoĭ zony Chernobyl’skoĭ atomnoĭ élektrostantsii . Mikrobiol. Z. 1996, 58, 3–12. (In Russian)
- Romanovskaia, V.A.; Sokolov, I.G.; Rokitko, P.V.; Chernaia, N.A. Ekologicheskie posledstviia radioaktivnogo zagriazneniia dlia pochvennykh bakteriĭ v 10-km zone ChAES . Mikrobiologiia 1998, 67, 274–280. (In Russian)
- Krivolutzkii, D.A.; Pokarzhevskii, A.D. Effects of radioactive fallout on soil animal populations in the 30 km zone of the Chernobyl atomic power station. Sci. Total Environ. 1992, 112, 69–77.
- Suzuki, J.; Egami, N. Mortality of the earthworms, Eisenia foetida, after γ-irradiation at different stages of their life history. J. Radiat. Res. 1983, 24, 209–220.
- Hertel-Aas, T.; Oughton, D.H.; Jaworska, A.; Bjerke, H.; Salbu, B.; Brunborg, G. Effects of chronic gamma irradiation on reproduction in the earthworm Eisenia fetida (Oligochaeta). Radiat. Res. 2007, 168, 515–526.
- Ihara, H.; Kumagai, A.; Hori, T.; Nanba, K.; Aoyagi, T.; Takasaki, M.; Katayama, Y. Direct comparison of bacterial communities in soils contaminated with different levels of radioactive cesium from the first Fukushima nuclear power plant accident. Sci. Total Environ. 2021, 756, 143844.
- Higo, M.; Kang, D.J.; Isobe, K. First report of community dynamics of arbuscular mycorrhizal fungi in radiocesium degradation lands after the Fukushima-Daiichi Nuclear disaster in Japan. Sci. Rep. 2019, 9, 8240.
- Bever, J.D.; Richardson, S.C.; Lawrence, B.M.; Holmes, J.; Watson, M. Preferential allocation to beneficial symbiont with spatial structure maintains mycorrhizal mutualism. Ecol. Lett. 2009, 12, 13–21.
- Barto, E.K.; Hilker, M.; Müller, F.; Mohney, B.K.; Weidenhamer, J.D.; Rillig, M.C. The fungal fast lane: Common mycorrhizal networks extend bioactive zones of allelochemicals in soils. PLoS ONE 2011, 6, e27195.
- Arrange, A.A.; Phelps, T.J.; Benoit, R.E.; Palumbo, A.V.; White, D.C. Bacterial sensitivity to UV light as a model for ionizing radiation resistance. J. Microbiol. Methods 1993, 18, 127–136.
- Kawaguchi, Y.; Shibuya, M.; Kinoshita, I.; Yatabe, J.; Narumi, I.; Shibata, H.; Hayashi, R.; Fujiwara, D.; Murano, Y.; Hashimoto, H.; et al. DNA damage and survival time course of deinococcal cell pellets during 3 years of exposure to outer space. Front. Microbiol. 2020, 11, 2050.
- Musilova, M.; Wright, G.; Ward, J.M.; Dartnell, L.R. Isolation of radiation-resistant bacteria from Mars analog Antarctic Dry Valleys by preselection, and the correlation between radiation and desiccation resistance. Astrobiology 2015, 15, 1076–1090.
- Geras’kin, S.A.; Dikarev, V.G.; Zyablitskaya, Y.Y.; Oudalova, A.A.; Spirin, Y.V. Cytogenetic effects of radiation on agricultural plants observed in the Chernobyl region during the first years after the accident. KURRI-KR 2002, 79, 287–296.
- Boratyński, Z.; Arias, J.; Garcia, C.; Mappes, T.; Mousseau, T.A.; Møller, A.P.; Pajares, A.J.M.; Piwczyński, M.; Tukalenko, E. Ionizing radiation from Chernobyl affects development of wild carrot plants. Sci. Rep. 2016, 6, 39282.
- Mousseau, T.A.; Milinevsky, G.; Kenney-Hunt, J.; Møller, A.P. Highly reduced mass loss rates and increased litter layer in radioactively contaminated areas. Oecologia 2014, 175, 429–437.
- van der Heijden, M.G.; Bardgett, R.D.; van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310.
- Dmitriev, A.; Guscha, N.; Dyachenko, A. Effects of low dose radiation on plant-pathogen interactions in Chernobyl Zone. Radioprotection 2008, 43, 148.
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic profiles of the creeping wood sorrel Oxalis corniculata in radioactively contaminated fields in Fukushima: Dose-dependent changes in key metabolites. Life 2022, 12, 115.
- Zhu, J.; Sun, X.; Zhang, Z.D.; Tang, Q.Y.; Gu, M.Y.; Zhang, L.J.; Hou, M.; Sharon, A.; Yuan, H.L. Effect of ionizing radiation on the bacterial and fungal endophytes of the halophytic plant Kalidium schrenkianum. Microorganisms 2021, 9, 1050.
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738.
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.I.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2018, 109, 206–211.
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 13232.
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-Ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67.
- Shimizu, M. Endophytic actinomycetes: Biocontrol agents and growth promoters. In Bacteria in Agrobiology: Pant Growth Responses; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–220.
- Taskaev, A.I.; Frolova, N.P.; Popova, O.N.; Shevchenko, V.A. The monitoring of herbaceous seeds in the 30-km zone of the Chernobyl nuclear accident. Sci. Total Environ. 1992, 112, 57–67.
- Imanaka, T. Chernobyl and Fukushima: Comparison of accident process and radioactive contamination. Kagaku 2016, 86, 0252–0257. (In Japanese)
- Ludovici, G.M.; Chierici, A.; de Souza, S.O.; d’Errico, F.; Iannotti, A.; Malizia, A. Effects of ionizing radiation on flora ten years after the Fukushima Dai-ichi disaster. Plants 2022, 11, 222.
- Yoshioka, A.; Mishima, Y.; Fukasawa, K. Pollinators and other flying insects inside and outside the Fukushima evacuation zone. PLoS ONE 2015, 10, e0140957.
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 15.
- Cannon, G.; Kiang, J.G. A review of the impact on the ecosystem after ionizing irradiation: Wildlife population. Int. J. Radiat. Biol. 2022, 98, 1054–1062.
- Nohara, C.; Hiyama, A.; Taira, W.; Otaki, J.M. Robustness and radiation resistance of the pale grass blue butterfly from radioactively contaminated areas: A possible case of adaptive evolution. J. Hered. 2018, 109, 188–198.
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240.
- Hinton, T.G.; Alexakhin, R.; Balonov, M.; Gentner, N.; Hendry, J.; Prister, B.; Strand, P.; Woodhead, D. Radiation-induced effects on plants and animals: Findings of the United Nations Chernobyl Forum. Health Phys. 2007, 93, 427–440.
- Adam-Guillermin, C.; Hertal-Aas, T.; Oughton, D.; Blanchard, L.; Alonzo, F.; Armant, O.; Horemans, N. Radiosensitivity and transgenerational effects in non-human species. Ann. ICRP 2018, 47, 327–341.
- Horemans, N.; Spurgeon, D.J.; Lecomte-Pradines, C.; Saenen, E.; Bradshaw, C.; Oughton, D.; Rasnaca, I.; Kamstra, J.H.; Adam-Guillermin, C. Current evidence for a role of epigenetic mechanisms in response to ionizing radiation in an ecotoxicological context. Environ. Pollut. 2019, 251, 469–483.
- Gombeau, K.; Bonzom, J.M.; Cavalié, I.; Camilleri, V.; Orjollet, D.; Dubourg, N.; Beaugelin-Seiller, K.; Bourdineaud, J.P.; Lengagne, T.; Armant, O.; et al. Dose-dependent genomic DNA hypermethylation and mitochondrial DNA damage in Japanese tree frogs sampled in the Fukushima Daiichi area. J. Environ. Radioact. 2020, 225, 106429.
- Hancock, S.; Vo, N.T.K.; Goncharova, R.I.; Seymour, C.B.; Byun, S.H.; Mothersill, C.E. One-Decade-Spanning transgenerational effects of historic radiation dose in wild populations of bank voles exposed to radioactive contamination following the chernobyl nuclear disaster. Environ. Res. 2020, 80, 108816.




