
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Elena Ramírez-Maldonado | -- | 2246 | 2024-02-16 19:50:02 | | | |
| 2 | Jessie Wu | + 29 word(s) | 2275 | 2024-02-26 09:46:59 | | |
Video Upload Options
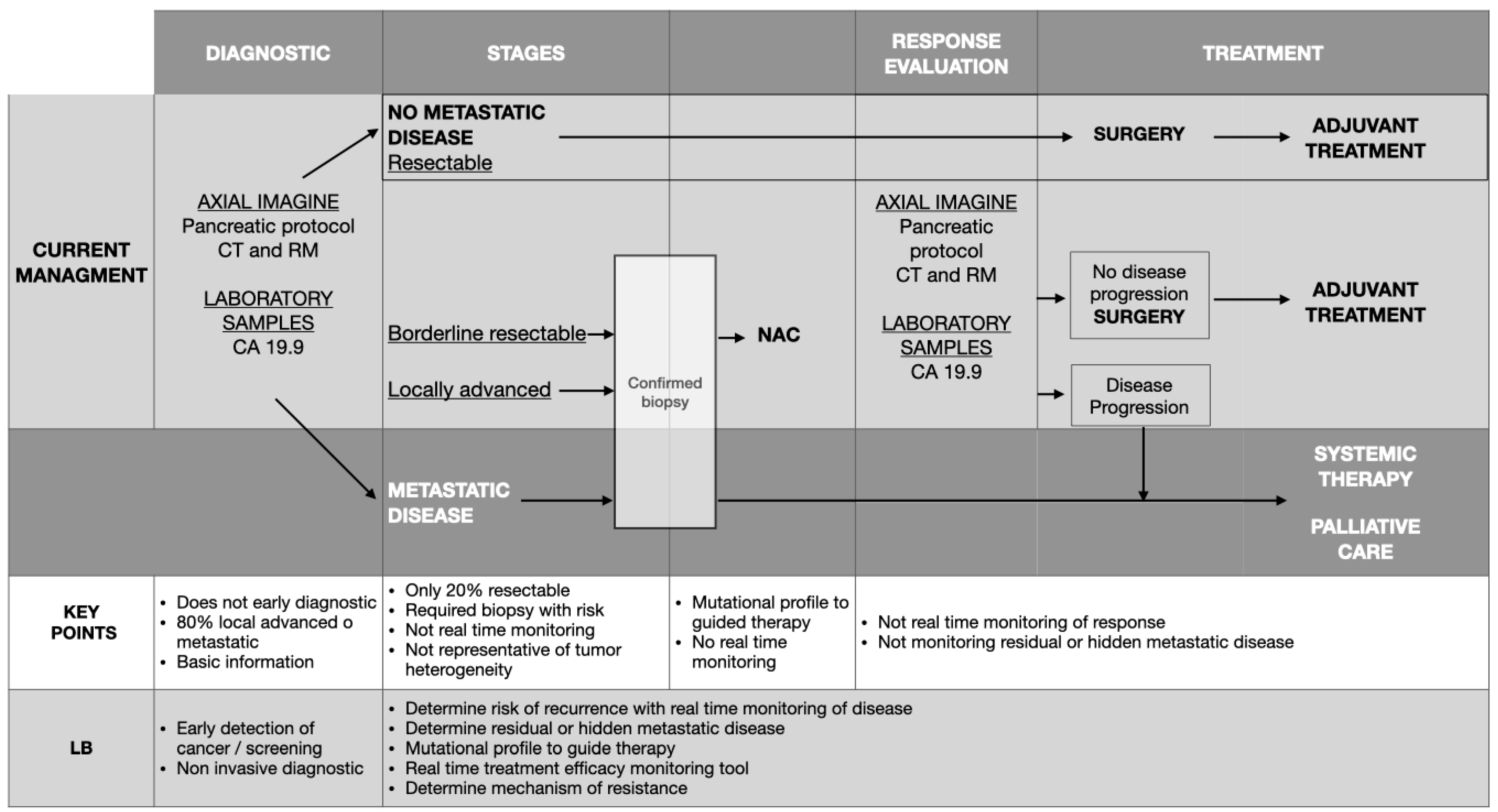
Pancreatic ductal adenocarcinoma contributes significantly to global cancer-related deaths, featuring only a 10% survival rate over five years. The quest for novel tumor markers is critical to facilitate early diagnosis and tailor treatment strategies for this disease, which is key to improving patient outcomes. In pancreatic ductal adenocarcinoma, these markers have been demonstrated to play a crucial role in early identification, continuous monitoring, and prediction of its prognosis and have led to better patient outcomes. Nowadays, biopsy specimens serve to ascertain diagnosis and determine tumor type. However, liquid biopsies present distinct advantages over conventional biopsy techniques. They offer a noninvasive, easily administered procedure, delivering insights into the tumor’s status and facilitating real-time monitoring. Liquid biopsies encompass a variety of elements, such as circulating tumor cells, circulating tumor DNA, extracellular vesicles, microRNAs, circulating RNA, tumor platelets, and tumor endothelial cells.
1. Impact of Liquid Biopsy in Early and Differential Diagnosis of Pancreatic Ductal Adenocarcinoma
| ctDNA | Circulating Tumor Cells (CTCs) | Extracellular Vesicles (EVs) | |
|---|---|---|---|
| Target | KRAS, TP53, CDKN2A, SMAD4, BRAF, PIK3CA, ADAMTS1, BNC1, 5MC, H2AZ, H2A1.1, H3K4me2, h2ak119ub | CD45, CEP8, CK, EpCAM | KRAS, TP53, RNA: miRNA, longRNA Proteins markers: EFGR, EPCAM, MUC-1, GPC-1, WNT2 |
| Isolation | Blood | Blood | Body fluids |
| Tumor information | Epigenetic information | DNA, RNA, Protein | DNA, RNA, Protein |
| Technological approaches | qPCR, dPCR, ddPCR, NGS, commercial kits | Immunoaffinity, Physical methods (size and density) | Density-based, size-based, affinity-based, commercial kits |
| Advantages | qPCR: Fast and low-cost dPCR: High sensitivity/Specificity NGS: capability to screen for a broad range of genetic variants using high DNA input |
Immunoaffinity: Specific, label-free obtained Physical methods: Fast, simple, Low-cost, label-free obtained |
Density-based: low cost. Independent of marker expression. Size-based: Low-cost, fast, Independent of marker expression. Affinity-based: Specificity. High purity. Commercial kits: Simple, fast. |
| Disadvantages | General: No early stages qPCR: Low sensitivity. Only points mutations. dPCR: High cost. Only points mutations. NGS: Variable sensitivity. High cost. |
General: Isolation complex and expensive. Technical variability Immunoaffinity: capture only one subpopulation. Low purity. Physical methods: Needs immuno-labeling techniques to distinguish CTCs |
General: Isolation complex by contamination and expensive Density-based: Time, high volume sample, can damage EVs. Size based: contamination. Affinity-based: low sample yield. Commercial kits: High cost. |
| Sensitivity (S) (%) | 34–71% KRAS mutations: codons 12, 13, 61, in different stages. |
73–76% CD45/CEP8 100% Mt, 58% resectable Anti-EpCAM portal vein Blood |
67% ES, 80% LA, 85% Mt KRAS mutations in exoDNA 50% ES GPC1 miRNAs Increased expression |
| Specificity (Sp) (%) | 75–81% Mutations KRAS exon 2 |
68% CD45/CEP8 |
90% ES GPC1 |
| Combined techniques (%) | S: 85–98%, Sp: 77–81% ctDNA (KRAS exon 2) with CA19.9 S: 47% ctDNA (KRAS MAFs) with CA19.9 |
S: 100%, Sp: 80% CTCs.with EVs |
NR |
| Application | No suitable for screening of PDAC Monitoring postoperative minimal residual disease Predictor of disease recurrence and prognosis |
Not present in healthy controls Variable sensitivity in early diagnosis Excellent specificity. Follow-up of disease recurrence and prognosis Functional analysis drug resistance |
The highest sensitivity and specificity in early detection Evaluated response of resection or any therapy Biotherapeutic application |
1.1. Circulating Tumor DNA
1.2. Circulating Tumor Cells
1.3. Extracellular Vesicles
1.4. miRNAs
2. Role of Liquid Biopsy after Resection of Pancreatic Ductal Adenocarcinoma
2.1. Circulating Tumor DNA
2.2. Circulating Tumor Cells
2.3. Extracellular Vesicles
References
- Heredia-Soto, V.; Rodríguez-Salas, N.; Feliu, J. Liquid biopsy in pancreatic cancer: Are we ready to apply it in the clinical practice? Cancers 2021, 13, 1986.
- Zhu, Y.; Zhang, H.; Chen, N.; Hao, J.; Jin, H.; Ma, X. Diagnostic value of various liquid biopsy methods for pancreatic cancer: A systematic review and meta-analysis. Medicine 2020, 99, e18581.
- Yang, J.; Li, S.; Li, J.; Wang, F.; Chen, K.; Zheng, Y.; Wang, J.; Lu, W.; Zhou, Y.; Yin, Q.; et al. A meta-analysis of the diagnostic value of detecting K-ras mutation in pancreatic juice as a molecular marker for pancreatic cancer. Pancratology 2016, 16, 605–614.
- Shi, C.; Fukushima, N.; Abe, T.; Bian, Y.; Hua, L.; Wendelburg, B.J.; Yeo, C.J.; Hruban, R.H.; Goggins, M.G.; Eshleman, J.R. Sensitive and quantitative detection of KRAS2 gene mutations in pancreatic duct juice differentiates patients with pancreatic cancer from chronic pancreatitis, potential for early detection. Cancer Biol. Ther. 2008, 7, 353–360.
- Hata, T.; Ishida, M.; Motoi, F.; Yamaguchi, T.; Naitoh, T.; Katayose, Y.; Egawa, S.; Unno, M. Telomerase activity in pancreatic juice differentiates pancreatic cancer from chronic pancreatitis: A meta-analysis. Pancreatology 2016, 16, 372–381.
- Zhang, L.; Farrell, J.J.; Zhou, H.; Elashowff, D.; Akin, D.; Park, N.H.; Chia, D.; Wong, D.T. Salivary transcriptomic biomarkers for detection of resectable pancreatic cancer. Gastroenterology 2010, 138, 949–957.
- Xie, Z.; Chen, X.; Li, J.; Guo, Y.; Li, H.; Pan, X.; Jiang, J.; Liu, H.; Wu, B. Salivary HOTAIR and PVT1 as novel biomarkers for early pancreatic cancer. Oncotarget 2016, 7, 25408–25419.
- Xie, Z.; Yin, X.; Gong, B.; Nice, W.; Wu, B.; Zhang, X.; Huang, J.; Zhang, P.; Zhou, Z.; Li, Z. Salivary microRNAs show potential as a noninvasive biomarker for detecting resectable pancreatic cancer. Cancer Prev. Res. 2015, 8, 165–173.
- Yoshizawa, N.; Sugimoto, K.; Tameda, M.; Inagaki, Y.; Ikejiri, M.; Inoue, H.; Usui, M.; Takei, Y. miR-3940-5p/miR-8069 ratio in urine exosomes is a novel diagnostic biomarker for pancreatic ductal adenocarcinoma. Once Lett. 2020, 19, 2677–2684.
- Terasawa, H.; Kinugasa, H.; Ako, S.; Hirai, M.; Matsushita, H.; Uchida, D.; Tomoda, T.; Matsumoto, K.; Horiguchi, S.; Kato, H.; et al. Utility of liquid biopsy using urine in patients with pancreatic ductal adenocarcinoma. Cancer Biol. Ther. 2019, 20, 1348–1353.
- Tempero, M.A.; Malafa, M.P.; Al-Hawari, M.; Behrman, S.W.; Benson, A.B.; Cardin, D.B.; Chiorean, E.G.; Chung, V.; Czito, B.; Del Chiaro, M.; et al. Pancreatic adenocarcinoma, Version 1.2024, NCCN Clinical practice guidelines in oncology. J. Natl. Cancer Netw. 2021, 19, 430–457.
- Watanabe, F.; Suzuki, K.; Noda, H.; Rikiyama, T. Liquid biopsy leads to a paradigm shift in the treatment of pancreatic cancer. World J. Gastroenterol. 2022, 28, 6478–6496.
- Rofi, E.; Vivaldi, C.; Del Re, M.; Arrigoni, E.; Crucitta, S.; Fuel, N.; Fogli, S.; Vasile, E.; Musettini, G.; Fornaro, L.; et al. The emerginn role of liquid biopsy in diagnosis, prognosis, an treatment monitoring of pancreatic cancer. Pharmacogenomics 2019, 20, 49–68.
- Raufi, A.G.; May, M.S.; Hadfield, M.J.; Seyhan, A.A.; El-Deiry, W.S. Advances in liquid biopsy technology and implications for pancreatic cancer. Int. J. Mol. Sci. 2023, 24, 4238.
- Adamo, P.; Cowley, C.M.; Neal, C.P.; Mistry, V.; Page, K.; Denninson, A.R.; Isherwood, J.; Hastings, R.; Luo, J.; Moore, D.A.; et al. Profiling tumour heterogeneity through circulating tumour DNA in patients with pancreatic cancer. Oncotarget 2017, 8, 87221–87233.
- Finkelstein, S.D.; Bibbo, M.; Loren, D.E.; Siddiqui, A.A.; Solomides, C.; Kowalski, T.E.; Ellsworth, E. Molecular analysis of centrifugation supernatant fluid from pancreaticobiliary duct samples can improve cancer detection. Acta Cytol. 2012, 56, 439–447.
- Sikora, K.; Bedin, C.; Vicentini, C.; Malpeli, G.; D’Angelo, E.; Sperandio, N.; Lawlor, R.T.; Bassi, C.; Tortora, G.; Nitti, D.; et al. Evaluation of cell-free DNA as a biomarker for pancreatic malignancies. Int. J. Biol. Markers 2015, 30, e136–e141.
- Grunvald, M.W.; Jacobson, R.A.; Kuzel, T.M.; Pappas, S.G.; Masood, A. Current status of circulating tumor DNA liquid biopsy in pancreatic cancer. Int. J. Mol. Sci. 2020, 21, 7651.
- Rhim, A.D.; Thege, F.I.; Santana, S.M.; Lannin, T.B.; Saha, T.N.; Tsai, S.; Maggs, L.R.; Kochman, M.L.; Gingsberg, G.G.; Lieb, J.G.; et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology 2014, 146, 647–651.
- Kulemann, B.; Pitman, M.B.; Liss, A.S.; Valsangkar, N.; Fernández-Del Castillo, C.; Lillemoe, K.D.; Hoeppner, J.; Mino-Kenudson, M.; Warshaw, A.L.; Thayer, S.P. Circulating tumor cells found in patients with localized and advanced pancreatic cancer. Pancreas 2015, 44, 547–550.
- Cauley, C.E.; Pitman, M.B.; Zhou, J.; Perkins, J.; Coleman, B.; Liss, A.S.; Fernández-Del Castillo, C.; Warshaw, A.L.; Lillemoe, K.D.; Ayer, S.P. Circulating Epithelial Cells in Patients with Pancreatic Lesions: Clinical and Pathologic Findings. J. Am. Coll. Surg. 2015, 221, 699–707.
- Qi, Z.H.; Xu, H.X.; Zhang, S.R.; Xu, J.Z.; Li, S.; Gao, H.L.; Jin, W.; Wang, W.Q.; Wu, C.T.; Ni, Q.X.; et al. The Significance of Liquid Biopsy in Pancreatic Cancer. J. Cancer 2018, 9, 3417–3426.
- Ankeny, J.S.; Court, C.M.; Hou, S.; Li, Q.; Song, M.; Wu, D.; Chen, J.F.; Lee, T.; Lin, M.; Sho, S.; et al. Circulating tumour cells as a biomarker for diagnosis and staging in pancreatic cancer. Br. J. Cancer 2016, 114, 1367–1375.
- Buscail, E.; Alix-Panabieres, C.; Quincy, P.; Cauvin, T.; Chauvet, A.; Degrandi, O.; Caumont, C.; Verdom, S.; Lamrissi, I.; Moranvillier, I.; et al. High Clinical Value of Liquid Biopsy to Detect Circulating Tumor Cells and Tumor Exosomes in Pancreatic Ductal Adenocarcinoma Patients Eligible for Up-Front Surgery. Cancers 2019, 11, 1656.
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scale, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747.
- Maire, F.; Micard, S.; Hammel, P.; Voitot, H.; Lévy, P.; Cugnenc, P.H.; Ruszniewski, P.; Puig, P.L. Differential diagnosis between chronic pancreatitis and pancreatic cancer: Value of the detection of KRAS2 mutations in circulating DNA. Br. J. Cancer 2002, 87, 551–554.
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gaming, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182.
- Zhang, J.; Zhu, Y.; Shi, J.; Zhang, K.; Zhang, Z.; Zhang, H. Sensitive Signal Amplifying a Diagnostic Biochip Based on a Biomimetic Periodic Nanostructure for Detecting Cancer Exosomes. ACS Appl. Mater. Interfaces 2020, 12, 33473–33482.
- Lewis, J.M.; Vyas, A.D.; Qiu, Y.; Messer, K.S.; White, R.; Heller, M.J. Integrated Analysis of Exosomal Protein Biomarkers on Alternating Current Electrokinetic Chips Enables Rapid Detection of Pancreatic Cancer in Patient Blood. ACS Nano 2018, 12, 3311–3320.
- Lux, A.; Kahlert, C.; Grützmann, R.; Pilarsky, C. c-Met and PD-l1 on circulating exosomes as diagnostic and prognostic markers for pancreatic cancer. Int. J. Mol. Sci. 2019, 20, 3305.
- Yan, T.B.; Huang, J.Q.; Huang, S.Y.; Ahir, B.K.; Li, L.M.; Mo, Z.N.; Zhong, J.H. Advances in the Detection of Pancreatic Cancer Through Liquid Biopsy. Front. Oncol. 2021, 11, 801173.
- Schultz, N.A.; Dehlendorff, C.; Jensen, B.V.; Bjerregaard, J.K.; Nielsen, K.P.; Bojesen, S.E.; Calatayud, D.; Nielsen, S.E.; Yilmaz, M.; Holländer, N.H.; et al. MicroRNA biomarkers in whole blood for detection of pancreatic cancer. JAMA 2014, 311, 392–404.
- Yan, Q.; Hu, D.; Li, M.; Chen, Y.; Wu, X.; Ye, Q.; Wang, Z.; He, L.; Zhu, J. The Serum MicroRNA Signatures for Pancreatic Cancer Detection and Operability Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 379.
- Komatsu, S.; Ichikawa, D.; Miyamae, M.; Kawaguchi, T.; Morimura, R.; Hirajima, S.; Okajima, W.; Ohashi, T.; Imamura, T.; Konishi, H.; et al. Malignant potential in pancreatic neoplasm; New insights provided by circulating miR-223 in plasma. Expert Opin. Biol. Ther. 2015, 15, 773–785.
- Bartsch, D.K.; Gercke, N.; Strauch, K.; Wieboldt, R.; Matthäi, E.; Wagner, V.; Rospleszcz, S.; Schäfer, A.; Franke, F.S.; Mintziras, I.; et al. The combination of miRNA-196b, LCN2, and TIMP1 is a potential set of circulating biomarkers for screening individuals at risk for familial pancreatic cancer. J. Clin. Med. 2018, 7, 295.
- Peng, C.; Wang, J.; Gao, W.; Huang, L.; Liu, Y.; Li, X.; Li, Z.; Yu, X. Meta-analysis of the diagnostic performance of circulating micrornas for pancreatic cancer. Int. J. Med. Sci. 2021, 18, 660–671.
- Wang, J.; Raimondo, M.; Guha, S.; Chen, J.; Diao, L.; Dong, X.; Wallacew, M.B.; Killary, A.M.; Frazier, M.L.; Woodward, T.A.; et al. Circulating microRNAs in pancreatic juice as candidate biomarkers of pancreatic cancer. J. Cancer 2014, 5, 696–705.
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Burnt, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary microRNA in pancreatic cancer patients. PLoS ONE 2015, 10, e0130996.
- Xiao, D.; Dong, Z.; Zhen, L.; Xia, G.; Huang, X.; Wang, T.; Geo, H.; Yang, B.; Xu, C.; Wu, W.; et al. Combined exosomal GPC1, CD82, and serum CA19-9 as multiplex targets: A specific, sensitive, and reproducible detection panel for the diagnosis of pancreaticcancer. Mol. Cancer Res. 2020, 18, 1300–1310.
- Pu, X.; Ding, G.; Wu, M.; Zhou, S.; Jia, S.; Cao, L. Elevated expression of exosomal microRNA–21 as a potential biomarker for the early diagnosis of pancreatic cancer using a tethered cationic lipoplex nanoparticle biochip. Oncol. Lett. 2020, 19, 2062–2070.
- Sefrioui, D.; Blanchard, F.; Toure, E.; Basile, P.; Beaussire, L.; Dolfus, C.; Perdix, A.; Pares, M.; Antonietti, M.; Iwanicki-Caron, I.; et al. Diagnostic value of CA19.9, circulating tumour DNA and circulating tumour cells in patients with solid pancreatic tumours. Br. J. Cancer 2017, 117, 1017–1025.
- Wu, H.; Guo, S.; Liu, X.; Li, Y.; Su, Z.; He, Q.; Liu, X.; Zhang, Z.; Yu, L.; Shi, X.; et al. Noninvasive detection of pancreatic ductal adenocarcinoma using the methylation signature of circulating tumour DNA. BMC Med. 2022, 20, 458.
- Xu, Y.; Qin, T.; Li, J.; Wang, X.; Gao, C.; Xu, C.; Hao, J.; Liu, J.; Gao, S.; Ren, H. Detection of circulating tumor cells using negative enrichment immunofluorescence and an in situ hybridization system in pancreatic cancer. Int. J. Mol. Sci. 2017, 18, 622.
- Nagai, M.; Sho, M.; Akahori, T.; Nakagawa, K.; Nakamura, K. Application of liquid biopsy for surgical management of pancreatic cancer. Ann. Gastroenterol. Surg. 2020, 4, 216–223.
- Vidal, L.; Pando, E.; Blanco, L.; Fabregat-Franco, C.; Castet, F.; Sierra, A.; Macarulla, T.; Balsells, J.; Charco, R.; Vivancos, A. Liquid biopsy after resection of pancreatic adenocarcinoma and its relation to oncological outcomes. Systematic review and meta-analysis. Cancer Treat. Rev. 2023, 120, 102604.
- Leon, S.A.; Shapiro, B.; Sklaroff, D.M.; Yaros, M.J. Free DNA in the Serum of Cancer Patients and the Effect of Therapy. Cancer Res. 1977, 37, 646–650.




