Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Teodor Ioan Bajeu | -- | 2376 | 2024-02-14 08:25:10 | | | |
| 2 | Mona Zou | Meta information modification | 2376 | 2024-02-14 08:43:04 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Bajeu, I.; Niculescu, A.; Scafa-Udriște, A.; Andronescu, E. Intrastent Restenosis. Encyclopedia. Available online: https://encyclopedia.pub/entry/55032 (accessed on 07 February 2026).
Bajeu I, Niculescu A, Scafa-Udriște A, Andronescu E. Intrastent Restenosis. Encyclopedia. Available at: https://encyclopedia.pub/entry/55032. Accessed February 07, 2026.
Bajeu, Ioan-Teodor, Adelina-Gabriela Niculescu, Alexandru Scafa-Udriște, Ecaterina Andronescu. "Intrastent Restenosis" Encyclopedia, https://encyclopedia.pub/entry/55032 (accessed February 07, 2026).
Bajeu, I., Niculescu, A., Scafa-Udriște, A., & Andronescu, E. (2024, February 14). Intrastent Restenosis. In Encyclopedia. https://encyclopedia.pub/entry/55032
Bajeu, Ioan-Teodor, et al. "Intrastent Restenosis." Encyclopedia. Web. 14 February, 2024.
Copy Citation
Restenosis refers to the narrowing of a blood vessel’s diameter following an angioplasty procedure. Intrastent restenosis (ISR) is a challenging medical problem. A meta-analysis showed that percutaneous coronary intervention (PCI) for ISR is associated with a higher incidence of adverse cardiac events than PCI for de novo lesions. This happens especially because of a higher incidence of risk-adjusted major adverse cardiac events compared with PCI for de novo lesions at a median of ≈30 months.
intrastent restenosis
bare-metal stents
drug-eluting stents
pathophysiology
intravascular imaging
risk factors
coronary artery disease
treatment
drug-eluting ballons
angiography
1. ISR—Definition, Incidence, and Pathophysiology
ISR is characterized by a progressive luminal constriction within the stent, predominantly manifesting within the timeframe of 3 to 12 months subsequent to stent angioplasty [1]. From a clinical perspective, this phenomenon manifests as recurrent angina or heart failure [2]. It is noteworthy that it may also manifest as acute myocardial infarction in approximately 10% of afflicted patients [3]. In contrast, intrastent thrombosis constitutes an acute thrombotic occlusion, representing a dire event with the potential for precipitating sudden cardiac death or protracted acute myocardial infarction [4]. Despite the early revascularization, the mortality at 6 months is very high in this case [1].
The incidence of ISR is contingent upon various factors, including the stent type, the intricacy of the stented lesions, and the presence of risk factors [5]. Significantly elevated restenosis rates are observed in instances characterized by lesion complexity, such as those involving small vessels, extended lesions, or bifurcation lesions [6]. Determining the incidence of ISR is challenging due to its dependence on a multifactorial and variable interplay of factors. During the period in which balloon angioplasties were used, restenosis rates ranged from 32% to 55% among all procedures, subsequently declining to a range of 17% to 41% [7][8][9][10] with the advent of bare-metal stents [11][12][13]. A pivotal achievement in addressing this complication was the adoption of DESs, leading to a reduction in restenosis rates to levels below 10% [14][15]. ISR seems to manifest more frequently in patients suffering from multivessel disease compared to those affected solely by single-vessel disease [16].
ISR refers to a condition characterized by a significant reduction, typically equal to or exceeding 50%, in the diameter of the coronary lumen [17].
The advent of coronary stents has substantively transformed the therapeutic landscape for individuals afflicted by both chronic coronary syndromes and acute coronary syndromes [18][19]. Nevertheless, the widespread adoption of percutaneous revascularization procedures has ushered in a novel pathological phenomenon: ISR. The pathophysiology of ISR is a complex process involving many cellular mechanisms and depends on a series of risk factors [20].
It is characterized by the re-narrowing of a coronary artery at the site of a previously implanted stent [21]. The predominant mechanism underlying ISR is characterized by tissue proliferation, commonly referred to as neointimal hyperplasia [22]. This process reflects an exaggerated homeostatic healing response triggered by the arterial wall injury incurred during stent implantation, as the literature delineates [23]. The causative factors implicated in this phenomenon encompass localized inflammation arising from mechanical injury to the intimal and medial layers. This subsequently drives an aggressive neointimal hyperplasia marked by the proliferation of smooth muscle cells and the deposition of extracellular matrix [24][25]. Moreover, hypersensitivity reactions to the metallic components and polymer constituents intrinsic to early-generation DESs have been established as recognized mechanisms contributing to neointimal hyperplasia [26].
Coronary atherosclerotic disease is most commonly managed through the intervention of angioplasty with stent placement, a therapeutic modality that, while lifesaving, has been associated with adverse events that curtail its enduring efficacy [27]. Technical inadequacies, exemplified by factors such as stent malposition or inadequate expansion, resulting in the establishment of laminar blood flow patterns, subsequently promote the development of neointimal hyperplasia [28]. After angioplasty with a stent, a prevailing concern in long-term progression is the occurrence of intrastent neo-atherosclerosis [29]. Notably, the utilization of old-generation bare-metal stents has been constrained in adherence to contemporary medical practice guidelines due to a notable incidence of ISR, which has historically ranged from 17% to 41% [2]. While the introduction of new-generation DESs has substantially reduced the rate of such complications [14], DES-related failures persist as a matter of concern, affecting up to 10% of all implanted stents [14][15].
As mentioned before, using BMSs leads to a high rate of restenosis that can reach 40%. The principal etiology of this complication is commonly attributed to the incremental expansion of the extracellular fibrotic matrix, resulting in a gradual reduction in the luminal diameter [14]. In the contemporary era marked by the utilization of DESs, the pathogenesis of ISR appears to be contingent upon several interrelated factors, including the host’s heightened material sensitivity, the suppression of healing processes due to antiproliferative medications, and the organism’s reaction to stent implantation [30][31] (Figure 1). The pathophysiology of ISR (Figure 2) appears to involve a complex cascade of events, including the hyperplasia of smooth muscle cells, the migration of pro-inflammatory cells, the recruitment of marrow progenitors, including bone-marrow-derived progenitor cells (BMPCs), and the proliferation of the extracellular matrix, as suggested by the existing literature [30][31]. Hence, the interplay between the systemic pro-inflammatory response and the concurrent local pro-inflammatory response induced by the endothelial disruption during stent implantation collectively orchestrates the vascular healing process [32]. This dynamic interaction culminates in forming a neointimal zone characterized by the proliferation of unregulated smooth muscle fibers, ultimately contributing to the clinical manifestation of restenosis [33]. At the local level, the injury induced by stent deployment initiates a multifaceted cascade of processes, commencing with endothelial disruption and subsequent exposure of the intimal layer, which imparts a prothrombotic effect [32]. This local inflammatory milieu subsequently triggers the release of cytokines and growth factors, activates platelets, and fosters the proliferation and migration of smooth muscle fibers. These alterations can culminate in two potential outcomes: vascular healing or pathological progression. The latter pathological process, in particular, may eventually predispose individuals to ISR. Endothelial activation, prompted by cellular injury induced by the mechanical impact of the stent, initiates a cascade of events, including platelet activation. Notably, platelets are often the primary cells to respond to stent placement [34][35].
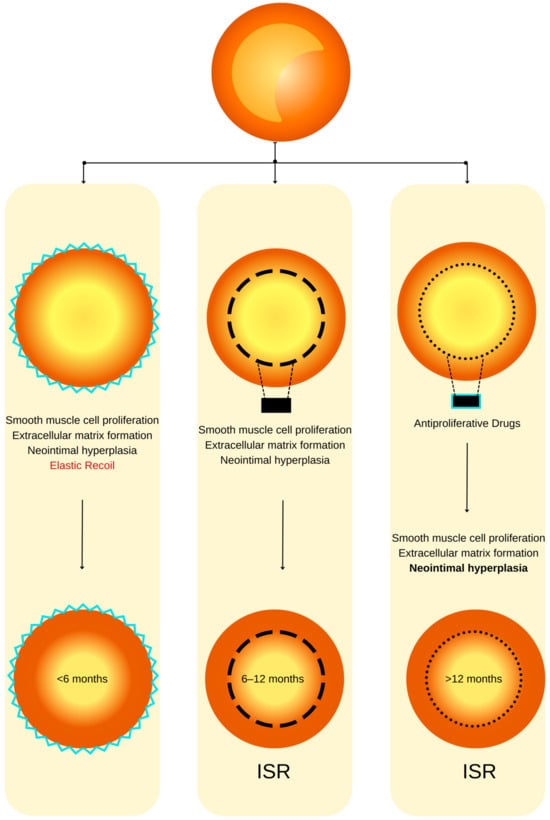
Figure 1. ISR physiopathology (adapted from an open–access source [26]). (Left): Coronary restenosis after conventional balloon angioplasty primarily arises from the phenomenon of elastic recoil of the arterial vessel wall. Moreover, the trauma inflicted on the coronary artery triggers the initiation of smooth muscle cell proliferation, their migration, and the deposition of an extracellular matrix. This cascade of events culminates in the formation of neointimal hyperplasia, which ultimately contributes to the pathogenesis of restenosis. (Center): The deployment of a BMS is associated with a heightened level of vascular injury, thereby augmenting the magnitude of neointimal hyperplasia and elevating the risk of in-stent restenosis. (Right): DESs dispense antiproliferative agents, effectively mitigating the extent of neointimal hyperplasia and correspondingly diminishing the susceptibility to in-stent restenosis.
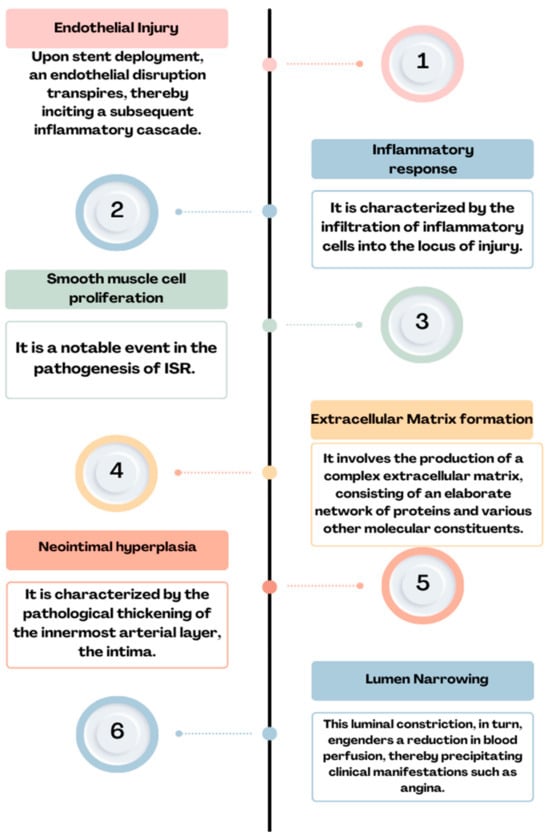
Figure 2. Physiopathology of ISR: endothelial injury→ inflammatory response→ smooth muscle cell proliferation→ extracellular matrix formation→ neointimal hyperplasia→ lumen narrowing = ISR.
After arterial injury, platelets promptly adhere to the affected site and initiate the release of thromboxane A2. The glycoprotein (GP) IIb/IIIa complex engages with fibrinogen, facilitating platelet aggregation and activation [36]. Furthermore, activated platelets release many bioactive factors, among which the platelet-derived growth factor (PDGF) is prominent. PDGF exhibits both mitogenic and chemotactic properties, significantly influencing smooth muscle cells [37]. It contributes to the induction of oxidative stress, thereby facilitating the transition of smooth muscle cells from a contractile phenotype to a synthetic one [38]. The release of histamine from mast cells and platelets has been implicated in the pathogenesis of intimal hyperplasia [38]. This assertion supports studies involving porcine models of endothelial dysfunction, wherein a substantial 20- to 90-fold elevation in histamine concentration has been documented [39]. Interleukin-1, when released by platelets, triggers an upregulation in the production of interleukin-6 and interleukin-8, exerting pro-inflammatory effects. This cascade of events includes the stimulation of smooth muscle fiber migration, achieved through the activation of actin polymerization and the initiation of tyrosine phosphorylation at the cytoskeletal protein level, particularly in association with focal adhesion and smooth muscle fiber proliferation [32]. Extracellular vesicles emanating from platelets elicit a response in smooth muscle fibers, inducing the production of interleukin-6 while concurrently triggering the expression of αIIbβ3 and P-selectin. These cellular changes promote interactions between smooth muscle fibers and monocytes [40].
Furthermore, platelet-derived microvesicles have been demonstrated to induce endothelial protein C receptor (EPCR) proliferation characterized by the presence of von Willebrand factor (vWF+) and CD34+ markers. This effect is mediated through the release of transforming growth factor (TGF)-β1, as evidenced in a rat arterial injury model [41].
Within the vessel wall, the coexistence of an inflammatory response stemming from the presence of the foreign body, coupled with the anti-inflammatory effect exerted by the drug released from the stent, collectively engenders a deceleration in the process of reendothelialization. The exposure of adhesion molecules, such as P-selectin, serves as a stimulus for the recruitment of corresponding monocytes and the subsequent secretion of pro-inflammatory cytokines, namely interleukins 6 and 8. This cascade of events culminates in the infiltration of neutrophils, monocytes, and macrophages into the subendothelial space [32]. Elevated levels of monocytes and eosinophils at the three-month mark following PCI serve as predictive factors for late ISR after deploying a pharmacologically active stent [42]. While the precise involvement of mast cells in the pathophysiology of restenosis remains a subject of ongoing investigation, it is noteworthy that mast cells have been observed to release chymase [43], an enzyme implicated in generating angiotensin II and tumor growth factor-β. This consequential cascade of molecular events subsequently fosters fibroblast proliferation and is associated with the development of neointimal formation [44][45]. Experimental evidence in animal models has demonstrated the efficacy of chymase expression inhibitors in attenuating neointimal formation [46].
Neo-atherosclerosis represents a mechanistic phenomenon observed in the context of DES utilization, and its incidence is on the rise. This process is typified by the accretion of lipid-laden foamy macrophages, occasionally accompanied by a necrotic core predominantly localized within the stent deployment region [29].
2. Risk Factors for ISR
2.1. Patient-Related Factors
The clinical prognosticators associated with in-stent restenosis (ISR) encompass a spectrum of factors, comprising, but not limited to, chronic kidney disease, elderly age, male gender, diabetes mellitus, and elevated body mass index, among various others [47]. Furthermore, established cardiovascular risk factors associated with atherosclerosis, such as hypertension, smoking, and dyslipidemia, have been evidenced to exert a pathogenetic influence on the development of neointimal hyperplasia [48].
2.2. Clinical Factors
ISR is a multifactorial phenomenon, subject to variability contingent upon individual patient characteristics, angiographic predictors, and the intricacies associated with the angioplasty procedure [49]. Notably, in the context of diabetic patients, it is imperative to underscore that the second generation of DESs exhibit a notable susceptibility to ISR, manifesting with an incidence rate of 8.7% [50]. In contrast, non-diabetic patients present a relatively diminished propensity for ISR, as evidenced by a prevalence of 5.7% [29].
Concerning gender-based prevalence, a study published in 2023 employed intracoronary imaging, specifically, optical coherence tomography (OCT), to demonstrate that the incidence of ISR exhibits a greater frequency among male patients [51]. Hence, the imaging data reveal a notable discrepancy, whereby men exhibit a significantly elevated risk in contrast to women, characterized by a higher incidence of thin-cap fibrous atherosclerosis (TCFA) (37.4% [n = 77] vs. 9.3% [n = 4], p < 0.001), as well as in-stent neo-atherosclerosis (ISNA) (82.0% [n = 169] vs. 62.8% [n = 27], p = 0.005).
Another study, conducted by Bajdechi et al. and published in 2023, explored a distinct patient cohort, specifically, individuals afflicted with Human Immunodeficiency Virus (HIV), revealing their heightened susceptibility to acute coronary events. This elevated risk can be attributed, in part, to the influence of antiviral medications and the concurrent discordant inflammatory response [52]. While patients afflicted with HIV exhibit an elevated risk of recurrent acute coronary syndrome, it is noteworthy that the stage of the disease does not exert a discernible influence on the prevalence of ISR [52].
Arterial hypertension, identified as an independent cardiovascular risk factor [53], was shown for the first time to be responsible for intrastent restenosis in a retrospective study involving 796 patients who underwent angiography due to angina recurrence or reversible myocardial ischemia [54]. The study revealed that effective blood pressure control during the initial PCI procedure correlated with a 24% lower risk of ISR. Additionally, factors such as total cholesterol levels, the use of beta-blockers or antiplatelet agents, and the site of stent implantation were linked to further reductions in ISR, particularly among patients with BMSs [54].
Moreover, it is well established that the cessation of antiplatelet medications ranks among the primary risk factors associated with intrastent restenosis. Consequently, non-adherence to pharmacological treatment regimens represents an additional significant risk factor for the development of ISR [55]. Additionally, individuals presenting with hypertension and a diagnosis of heart failure manifest an elevated susceptibility to ISR [56].
Furthermore, in a retrospective observational study, the incidence of restenosis in the coronary stent group was higher compared to the non-stent group when considering factors such as a family history of CHD, a history of type 2 diabetes, hypertension, smoking, drinking, withdrawal of aspirin, use of conventional doses of statins, calcified lesions, having ≥3 implanted stents, stent length ≥30 mm, and stent diameter <3 mm [57].
2.3. Angiographic Factors
As evidenced by the existing literature, the deployment of a stent in a vessel elicits a localized inflammatory response. This inflammatory cascade constitutes a pivotal component in the genesis of a pathological phenomenon recognized as ISR [58]. Furthermore, the intricacies of this multifaceted phenomenon extend beyond the mere presence of a stent, encompassing an array of stent-dependent, intra-stent, and extra-stent risk factors. The schematic delineation portrayed in Figure 3 illustrates the angiographic patterns under discussion. It is imperative to underscore that this classification holds pivotal significance in prognosis and expeditious patient triage for both clinical and investigative objectives [59].
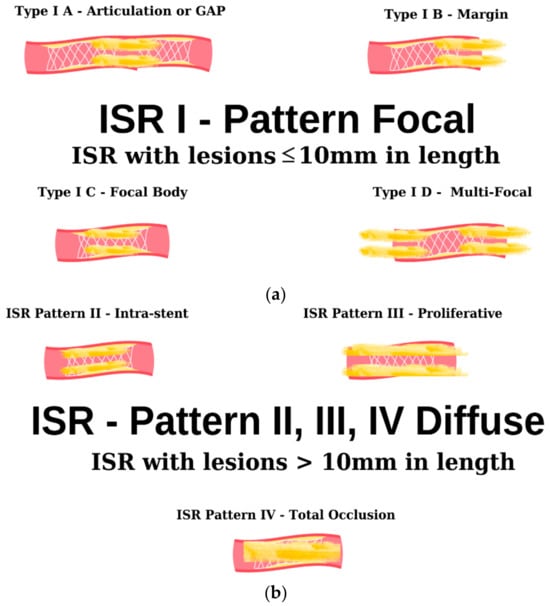
Figure 3. Angiographic classification of intrastent restenosis (adapted from an open-access source [59]). (a) Type I: there are four types of focal ISR described in the image above. (b) Type II: the observed lesions are confined strictly to the confines of the stent and do not exhibit any extension beyond its proximal or distal extremities. Type III: Diffuse, proliferative ISR. The identified lesions manifest a length exceeding 10 mm, exhibiting an extension that surpasses the boundaries of the stent at both its proximal and distal margins. Type IV: total occlusion of the stent resulting in no coronary perfusion.
In a retrospective study [60], the utilization of BMSs was associated with an ISR rate of 30%. Subsequently, with the advent of second-generation DESs, this proportion exhibited a notable reduction, declining to 12%. Consequently, the use of BMSs has been identified as a risk factor for ISR, thereby underscoring the rationale behind its diminishing prevalence in contemporary clinical practice in favor of the more favored DESs.
References
- Coronary Artery Stent Thrombosis: Clinical Presentation and Management—UpToDate. Available online: https://www.uptodate.com/contents/coronary-artery-stent-thrombosis-clinical-presentation-and-management/print (accessed on 25 November 2023).
- Buccheri, D.; Piraino, D.; Andolina, G.; Cortese, B. Understanding and managing in-stent restenosis: A review of clinical data, from pathogenesis to treatment. J. Thorac. Dis. 2016, 8, E1150.
- Ullrich, H.; Olschewski, M.; Münzel, T.; Gori, T. Coronary In-Stent Restenosis: Predictors and Treatment. Dtsch. Arztebl. Int. 2021, 118, 637.
- Modi, K.; Soos, M.P.; Mahajan, K. Stent Thrombosis; StatPearls Publishing LLC: Treasure Island, FL, USA, 2023.
- Alexandrescu, D.-M.; Mitu, O.; Costache, I.I.; Macovei, L.; Mitu, I.; Alexandrescu, A.; Arsenescu Georgescu, C. Risk factors associated with intra-stent restenosis after percutaneous coronary intervention. Exp. Ther. Med. 2021, 22, 1141.
- Li, M.; Hou, J.; Gu, X.; Weng, R.; Zhong, Z.; Liu, S. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur. J. Med. Res. 2022, 27, 12.
- Fishman, R.F.; Kuntz, R.E.; Carrozza, J.P.; Miller, M.J.; Senerchia, C.C.; Schnitt, S.J.; Diver, D.J.; Safian, R.D.; Baim, D.S. Long-term results of directional coronary atherectomy: Predictors of restenosis. J. Am. Coll. Cardiol. 1992, 20, 1101–1110.
- Le Feuvre, C.; Bonan, R.; Lespérance, J.; Gosselin, G.; Joyal, M.; Crépeau, J. Predictive factors of restenosis after multivessel percutaneous transluminal coronary angioplasty. Am. J. Cardiol. 1994, 73, 840–844.
- Bakhai, A.; Booth, J.; Delahunty, N.; Nugara, F.; Clayton, T.; McNeill, J.; Davies, S.W.; Cumberland, D.C.; Stables, R.H.; SV Stent Investigators. The SV stent study: A prospective, multicentre, angiographic evaluation of the BiodivYsio phosphorylcholine coated small vessel stent in small coronary vessels. Int. J. Cardiol. 2005, 102, 95–102.
- Agostoni, P.; Valgimigli, M.; Biondi-Zoccai, G.G.L.; Abbate, A.; Garcia Garcia, H.M.; Anselmi, M.; Turri, M.; McFadden, E.P.; Vassanelli, C.; Serruys, P.W.; et al. Clinical effectiveness of bare-metal stenting compared with balloon angioplasty in total coronary occlusions: Insights from a systematic overview of randomized trials in light of the drug-eluting stent era. Am. Heart J. 2006, 151, 682–689.
- Stettler, C.; Wandel, S.; Allemann, S.; Kastrati, A.; Morice, M.C.; Schömig, A.; Pfisterer, M.E.; Stone, G.W.; Leon, M.B.; de Lezo, J.S.; et al. Outcomes associated with drug-eluting and bare-metal stents: A collaborative network meta-analysis. Lancet 2007, 370, 937–948.
- Kastrati, A.; Mehilli, J.; Pache, J.; Kaiser, C.; Valgimigli, M.; Kelbæk, H.; Menichelli, M.; Sabaté, M.; Suttorp, M.J.; Baumgart, D.; et al. Analysis of 14 trials comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 2007, 356, 1030–1039.
- García del Blanco, B.; Rumoroso Cuevas, J.R.; Hernández Hernández, F.; Trillo Nouche, R. Spanish cardiac catheterization and coronary intervention registry. 22nd official report of the Spanish Society of Cardiology Working Group on Cardiac Catheterization and Interventional Cardiology (1990–2012). Rev. Esp. Cardiol. (Engl. Ed.) 2013, 66, 894–904.
- Moses, J.W.; Leon, M.B.; Popma, J.J.; Fitzgerald, P.J.; Holmes, D.R.; O’Shaughnessy, C.; Caputo, R.P.; Kereiakes, D.J.; Williams, D.O.; Teirstein, P.S.; et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N. Engl. J. Med. 2003, 349, 1315–1323.
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231.
- Zhao, L.P.; Xu, W.T.; Wang, L.; Li, H.; Shao, C.L.; Gu, H.B.; Chan, S.P.; Xu, H.F.; Yang, X.J. Influence of insulin resistance on in-stent restenosis in patients undergoing coronary drug-eluting stent implantation after long-term angiographic follow-up. Coron. Artery Dis. 2015, 26, 5–10.
- Chiastra, C.; Migliavacca, F. Modeling of Blood Flow in Stented Coronary Arteries. In Heat Transfer and Fluid Flow in Biological Processes; Academic Press: Cambridge, MA, USA, 2015; pp. 335–370.
- Bertolone, D.T.; Gallinoro, E.; Esposito, G.; Paolisso, P.; Bermpeis, K.; De Colle, C.; Fabbricatore, D.; Mileva, N.; Valeriano, C.; Munhoz, D.; et al. Contemporary Management of Stable Coronary Artery Disease. High Blood Press. Cardiovasc. Prev. 2022, 29, 207.
- Pham, V.; Moroni, A.; Gall, E.; Benedetti, A.; Zivelonghi, C.; Picard, F. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J. Clin. Med. 2023, 12, 2833.
- Shlofmitz, E.; Iantorno, M.; Waksman, R. Restenosis of Drug-Eluting Stents A New Classification System Based on Disease Mechanism to Guide Treatment and State-of-the-Art Review Circulation: Cardiovascular Interventions. Circ. Cardiovasc. Interv. 2019, 12, 7023.
- Lee, M.S.; Banka, G. In-stent Restenosis. Interv. Cardiol. Clin. 2016, 5, 211–220.
- Zain, M.A.; Jamil, R.T.; Siddiqui, W.J. Neointimal Hyperplasia; StatPearls: Treasure Island, FL, USA, 2023.
- Dangas, G.D.; Claessen, B.E.; Caixeta, A.; Sanidas, E.A.; Mintz, G.S.; Mehran, R. In-stent restenosis in the drug-eluting stent era. J. Am. Coll. Cardiol. 2010, 56, 1897–1907.
- Nolan, D.R.; Lally, C. An investigation of damage mechanisms in mechanobiological models of in-stent restenosis. J. Comput. Sci. 2018, 24, 132–142.
- He, R.; Zhao, L.; Silberschmidt, V.V.; Liu, Y. Mechanistic evaluation of long-term in-stent restenosis based on models of tissue damage and growth. Biomech. Model Mechanobiol. 2020, 19, 1425–1446.
- Torrado, J.; Buckley, L.; Durán, A.; Trujillo, P.; Toldo, S.; Valle Raleigh, J.; Abbate, A.; Biondi-Zoccai, G.; Guzmán, L.A. Restenosis, Stent Thrombosis, and Bleeding Complications: Navigating between Scylla and Charybdis. J. Am. Coll. Cardiol. 2018, 71, 1676–1695.
- Timmis, A.; Townsend, N.; Gale, C.; Grobbee, R.; Maniadakis, N.; Flather, M.; Wilkins, E.; Wright, L.; Vos, R.; Bax, J.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2017. Eur. Heart J. 2018, 39, 508–577.
- Ng, J.C.K.; Lian, S.S.; Zhong, L.; Collet, C.; Foin, N.; Ang, H.Y. Stent malapposition generates stent thrombosis: Insights from a thrombosis model. Int. J. Cardiol. 2022, 353, 43–45.
- Nusca, A.; Viscusi, M.M.; Piccirillo, F.; De Filippis, A.; Nenna, A.; Spadaccio, C.; Nappi, F.; Chello, C.; Mangiacapra, F.; Grigioni, F.; et al. In Stent Neo-Atherosclerosis: Pathophysiology, Clinical Implications, Prevention, and Therapeutic Approaches. Life 2022, 12, 393.
- Virmani, R.; Farb, A. Pathology of in-stent restenosis. Curr. Opin. Lipidol. 1999, 10, 499–506.
- Yutani, C.; Ishibashi-Ueda, H.; Suzuki, T.; Kojima, A. Histologic evidence of foreign body granulation tissue and de novo lesions in patients with coronary stent restenosis. Cardiology 1999, 92, 171–177.
- Clare, J.; Ganly, J.; Bursill, C.A.; Sumer, H.; Kingshott, P.; de Haan, J.B. The Mechanisms of Restenosis and Relevance to Next Generation Stent Design. Biomolecules 2022, 12, 430.
- Marx, S.O.; Totary-Jain, H.; Marks, A.R. Vascular Smooth Muscle Cell Proliferation in Restenosis. Circ. Cardiovasc. Interv. 2011, 4, 104–111.
- Becker, R.C.; Sexton, T.; Smyth, S.S. Translational Implications of Platelets as Vascular First Responders. Circ. Res. 2018, 122, 506–522.
- Hytönen, J.; Leppänen, O.; Braesen, J.H.; Schunck, W.H.; Mueller, D.; Jung, F.; Mrowietz, C.; Jastroch, M.; Von Bergwelt-Baildon, M.; Kappert , K.; et al. Activation of Peroxisome Proliferator-Activated Receptor-δ as Novel Therapeutic Strategy to Prevent In-Stent Restenosis and Stent Thrombosis. Arter. Thromb. Vasc. Biol. 2016, 36, 1534–1548.
- Reinthaler, M.; Jung, F.; Landmesser, U.; Lendlein, A. Trend to move from permanent metals to degradable, multifunctional polymer or metallic implants in the example of coronary stents. Expert. Rev. Med. Devices 2016, 13, 1001–1003.
- Factors Inducing In-Stent Restenosis: An In-Vitro Model|Request PDF. Available online: https://www.researchgate.net/publication/8246560_Factors_inducing_in-stent_restenosis_An_in-vitro_model (accessed on 25 November 2023).
- Jung, F.; Raghunath, M.; Blocki, A.; Gori, T. Restenosis after Coronary Stent Implantation: Cellular Mechanisms and Potential of Endothelial Progenitor Cells (A Short Guide for the Interventional Cardiologist). Cells 2022, 11, 2094.
- Fang, Y.I.; Namiki, H.; Tsunoda, E.; Shioda, S.; Shibata, M.; Nakatani, M.; Katagiri, T.; Takeyama, Y.; Ohata, H.; Honda, K.; et al. Marked increase in the histamine content of neointima after stent implantation of pig coronary artery and growth-promoting effects of histamine in cultured smooth muscle cells. Life Sci. 2005, 77, 241–251.
- Vajen, T.; Benedikter, B.J.; Heinzmann, A.C.A.; Vasina, E.M.; Henskens, Y.; Parsons, M.; Maguire, P.B.; Stassen, F.R.; Heemskerk, J.W.M.; Schurgers, L.J.; et al. Platelet extracellular vesicles induce a pro-inflammatory smooth muscle cell phenotype. J. Extracell. Vesicles 2017, 6, 1322454.
- Yan, J.; Bao, H.; Fan, Y.J.; Jiang, Z.L.; Qi, Y.X.; Han, Y. Platelet-derived microvesicles promote endothelial progenitor cell proliferation in intimal injury by delivering TGF-β1. FEBS J. 2020, 287, 5196–5217.
- Li, S.; Qiu, H.; Lin, Z.; Fan, L.; Guo, Y.; Zhang, Y.; Chen, L. The Early Predictive Value of Circulating Monocytes and Eosinophils in Coronary DES Restenosis. Front. Cardiovasc. Med. 2022, 9, 764622.
- Kennedy, S.; Wu, J.; Wadsworth, R.M.; Lawrence, C.E.; Maffia, P. Mast cells and vascular diseases. Pharmacol. Ther. 2013, 138, 53–65.
- Niccoli, G.; Montone, R.A.; Sabato, V.; Crea, F. Role of Allergic Inflammatory Cells in Coronary Artery Disease. Circulation 2018, 138, 1736–1748.
- Takai, S.; Miyazaki, M. Development and Application of Chymase Inhibitors: Effect of Chymase Inhibitor on Vascular Proliferation. Jpn. J. Pharmacol. 2002, 90, 223–227.
- Zhang, C.; Zhang, B.; Wang, H.; Tao, Q.; Ge, S.; Zhai, Z. Tumor necrosis factor alpha-stimulated gene-6 (TSG-6) inhibits the inflammatory response by inhibiting the activation of P38 and JNK signaling pathway and decreases the restenosis of vein grafts in rats. Heart Vessel. 2017, 32, 1536–1545.
- Pelliccia, F.; Zimarino, M.; Niccoli, G.; Morrone, D.; De Luca, G.; Miraldi, F.; De Caterina, R. In-stent restenosis after percutaneous coronary intervention: Emerging knowledge on biological pathways. Eur. Heart J. Open 2023, 3, oead083.
- Klein, L.W.; Nathan, S.; Maehara, A.; Messenger, J.; Mintz, G.S.; Ali, Z.A.; Rymer, J.; Sandoval, Y.; Al-Azizi, K.; Mehran, R.; et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. J. Soc. Cardiovasc. Angiogr. Interv. 2023, 2, 100971.
- Ninno, F.; Tsui, J.; Balabani, S.; Díaz-Zuccarini, V. A systematic review of clinical and biomechanical engineering perspectives on the prediction of restenosis in coronary and peripheral arteries. JVS Vasc. Sci. 2023, 4, 100128.
- Iqbal, J.; Serruys, P.W.; Silber, S.; Kelbaek, H.; Richardt, G.; Morel, M.A.; Negoita, M.; Buszman, P.E.; Windecker, S. Comparison of zotarolimus- and everolimus-eluting coronary stents: Final 5-year report of the RESOLUTE all-comers trial. Circ. Cardiovasc. Interv. 2015, 8, e002230.
- Yuan, X.; Jiang, M.; Feng, H.; Han, Y.; Zhang, X.; Chen, Y.; Gao, L. The effect of sex differences on neointimal characteristics of in-stent restenosis in drug-eluting stents: An optical coherence tomography study. Heliyon 2023, 9, e19073.
- Bajdechi, M.; Gurghean, A.; Bataila, V.; Scafa-Udriște, A.; Bajdechi, G.E.; Radoi, R.; Oprea, A.C.; Chioncel, V.; Mateescu, I.; Zekra, L.; et al. Particular Aspects Related to CD4+ Level in a Group of HIV-Infected Patients and Associated Acute Coronary Syndrome. Diagnostics 2023, 13, 2682.
- Hajar, R. Risk Factors for Coronary Artery Disease: Historical Perspectives. Heart Views 2017, 18, 109.
- Tocci, G.; Barbato, E.; Coluccia, R.; Modestino, A.; Pagliaro, B.; Mastromarino, V.; Giovannelli, F.; Berni, A.; Volpe, M. Blood Pressure Levels at the Time of Percutaneous Coronary Revascularization and Risk of Coronary In-Stent Restenosis. Am. J. Hypertens. 2016, 29, 509.
- Alexandrescu, D.; Crisan, A.; Mitu, O.; Macovei, L.; Costache, I.I.; Ivona, M.I.; Frasinariu, O.; Alexandrescu, A.; Georgescu, C.A. Antiplatelet Therapy and Inflammatory Status Associated with Intra Stent Restenosis after Percutaneous Coronary Intervention. Med.-Surg. J. 2021, 125, 335–342. Available online: https://www.revmedchir.ro/index.php/revmedchir/article/view/2446 (accessed on 25 November 2023).
- Singh, M.; Gersh, B.J.; McClelland, R.L.; Ho, K.K.L.; Willerson, J.T.; Penny, W.F.; Holmes, D.R. Clinical and angiographic predictors of restenosis after percutaneous coronary intervention: Insights from the Prevention of Restenosis with Tranilast and Its Outcomes (PRESTO) trial. Circulation 2004, 109, 2727–2731.
- Zhang, J.; Zhang, Q.; Zhao, K.; Bian, Y.J.; Liu, Y.; Xue, Y.T. Risk factors for in-stent restenosis after coronary stent implantation in patients with coronary artery disease: A retrospective observational study. Medicine 2022, 101, E31707.
- Ochijewicz, D.; Tomaniak, M.; Opolski, G.; Kochman, J. Inflammation as a determinant of healing response after coronary stent implantation. Int. J. Cardiovasc. Imaging 2021, 37, 791.
- Mehran, R.; Dangas, G.; Abizaid, A.S.; Mintz, G.S.; Lansky, A.J.; Satler, L.F.; Pichard, A.D.; Kent, K.M.; Stone, G.W.; Leon, M.B. Angiographic Patterns of In-Stent Restenosis. Circulation 1999, 100, 1872–1878.
- Cassese, S.; Byrne, R.A.; Tada, T.; Pinieck, S.; Joner, M.; Ibrahim, T.; King, L.A.; Fusaro, M.; Laugwitz, K.L.; Kastrati, A. Incidence and predictors of restenosis after coronary stenting in 10 004 patients with surveillance angiography. Heart 2014, 100, 153–159.
More
Information
Subjects:
Cardiac & Cardiovascular Systems
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
539
Revisions:
2 times
(View History)
Update Date:
16 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




