Your browser does not fully support modern features. Please upgrade for a smoother experience.

Submitted Successfully!
Thank you for your contribution! You can also upload a video entry or images related to this topic.
For video creation, please contact our Academic Video Service.
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Katarzyna Pacyga | -- | 1349 | 2024-02-13 16:35:16 | | | |
| 2 | Catherine Yang | Meta information modification | 1349 | 2024-02-17 04:09:58 | | |
Video Upload Options
We provide professional Academic Video Service to translate complex research into visually appealing presentations. Would you like to try it?
Cite
If you have any further questions, please contact Encyclopedia Editorial Office.
Pacyga, K.; Pacyga, P.; Topola, E.; Viscardi, S.; Duda-Madej, A. Natural Antimicrobial Agents for Wound Infections Treatment. Encyclopedia. Available online: https://encyclopedia.pub/entry/55023 (accessed on 07 February 2026).
Pacyga K, Pacyga P, Topola E, Viscardi S, Duda-Madej A. Natural Antimicrobial Agents for Wound Infections Treatment. Encyclopedia. Available at: https://encyclopedia.pub/entry/55023. Accessed February 07, 2026.
Pacyga, Katarzyna, Paweł Pacyga, Ewa Topola, Szymon Viscardi, Anna Duda-Madej. "Natural Antimicrobial Agents for Wound Infections Treatment" Encyclopedia, https://encyclopedia.pub/entry/55023 (accessed February 07, 2026).
Pacyga, K., Pacyga, P., Topola, E., Viscardi, S., & Duda-Madej, A. (2024, February 13). Natural Antimicrobial Agents for Wound Infections Treatment. In Encyclopedia. https://encyclopedia.pub/entry/55023
Pacyga, Katarzyna, et al. "Natural Antimicrobial Agents for Wound Infections Treatment." Encyclopedia. Web. 13 February, 2024.
Copy Citation
The rising prevalence of drug-resistant bacteria underscores the need to search for innovative and nature-based solutions. One of the approaches may be the use of plants that constitute a rich source of miscellaneous compounds with a wide range of biological properties. Natural products are one of the most promising and intensively examined agents to combat the consequences of the overuse and misuse of classical antibiotics.
plants
bioactive compounds
antibacterial activity
biological activity
1. Introduction
Inflammation is a complex disease process caused by endo- or exogenous factors, which can be biological (e.g., bacteria, viruses, fungi, protozoa, endotoxins, exotoxins), physical (e.g., mechanical, ionising radiation, magnetic field, ultrasound waves), or chemical (e.g., acids, bases) [1]. In the process of an inflammatory reaction, cells of the immune system (e.g., macrophages, neutrophils, monocytes, lymphocytes, mast cells), connective tissue cells (e.g., eicosanoids, i.e., prostaglandins, leukotrienes, and lipoxins), certain blood proteins (e.g., complement components, kinins, histamine, serotonin), and blood vessels are involved [2]. Additionally, adhesion molecules, responsible for interactions between leukocytes and endothelial cells, are involved in the pathomechanism of the inflammatory response. These include selectins (P and E-present on the epithelial surface, L-present on the surface of leukocytes), immunoglobulins (ICAM-intercellular, VCAM-vascular), integrins, and mucin-like glycoproteins [3][4]. The expression of these adhesion molecules is dependent on the presence of inflammatory mediators in the environment, i.e., histamine, platelet activating factor (PAF), IL-1, and TNF-α [5]. Recognition of the relevant adhesion molecules initiates the body’s response to the inflammatory agent, which can take the form of (1) cellular (T-lymphocyte involvement)-directed mostly against intracellular pathogens, (2) humoral (B-lymphocyte involvement)-protecting mainly against extracellular microorganisms, and (3) hemostatic (blood component involvement)-leading to platelet aggregation, clot formation, and intravascular coagulation.
Bacterial resistance is related to the adaptation and survival of the microorganism to a new environment in the presence of an antibiotic. Bacteria can: (1) block the penetration of a drug into a bacterial cell (e.g., resistance to β-lactam antibiotics, tetracyclines); (2) prevent the drug from reaching its target site in the cell (e.g., resistance to fluoroquinolones, tetracyclines); (3) modify the drug’s target site (e.g., resistance to β-lactam antibiotics, fluoroquinolones, macrolides, aminoglycosides, glycopeptides); and (4) inactivate or alter the drug by producing special enzymes (e.g., resistance to β-lactam antibiotics, aminoglycosides, chloramphenicol) [6]. In most cases, resistance is genetically determined. Spontaneous point mutations and recombination of genetic material can lead to resistant strains. In addition, this material can be transferred between microorganisms by (1) transformation (acquisition and incorporation of a free DNA fragment into the genome), (2) transduction (introduction of resistance genes by a bacteriophage), and (3) conjugation (transfer of plasmid-extra-chromosomal genetic material and transposons-fragments of DNA capable of movement) [7]. In these ways, resistance genes, not only between pathogenic bacteria but also their reservoirs, can become representatives of the microbiome.
2. Berberine (BRB)
Berberine (Figure 1) is a plant metabolite that belongs to the group of isoquinoline alkaloids and exhibits low toxicity as well as strong biological and pharmacological activities [8][9]. It is a very important natural alkaloid that is used to synthesise various bioactive derivatives by modifying functional groups that are important for the development of new and selective treatments [10]. Among the Berberidaceae family, the genus Berberis includes approximately 450–500 species, which are the major natural sources of berberine [9][10]. This active compound can be found in the leaves, stems, twigs, barks, rhizomes, and roots of many medicinal plants [10]. At present, berberine is of great interest due to its, among others, anticancer [11][12], cholesterol-lowering [13], antidiabetic [14], anti-obesity [15], hypolipidemic [16], hepatoprotective [17], antihypertensive [18], antidepressant [19], anti-inflammatory [20], antidiarrheal [21], anti-neurological [22][23], antibacterial [24], and antiviral [25] properties. However, the favourable applications of berberine are restricted by the weak pharmacokinetics of the compound. Nonetheless, berberine appears to be very effective and can be used in combination with other chemotherapeutics or treatments [8]. The growing interest in this compound can be seen in the increasing number of articles (Figure 2).

Figure 1. Berberine structure (ChemSpider database).
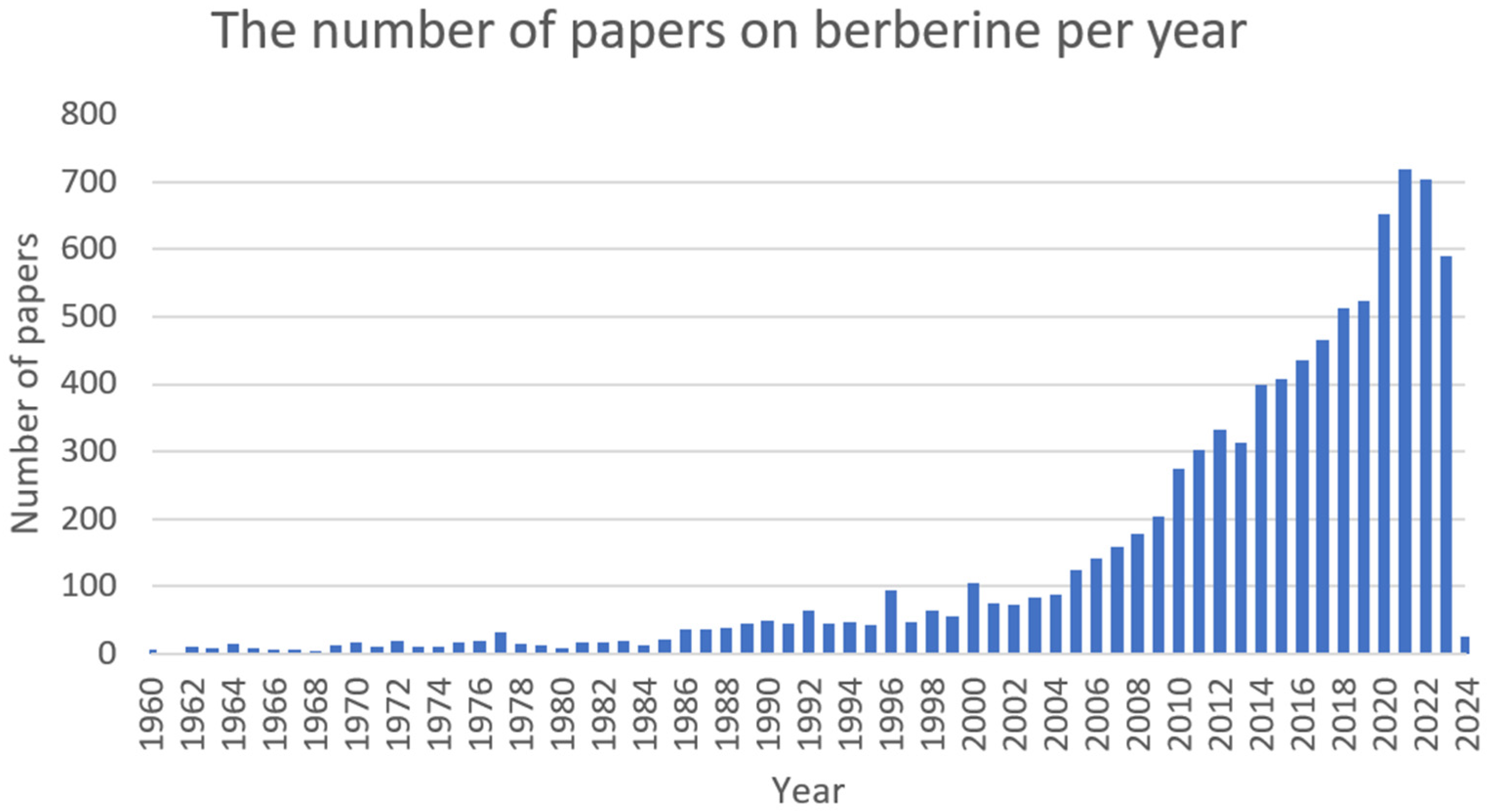
Figure 2. The number of papers on berberine per year (PubMed database).
3. Catechin (CT)
Catechins (Figure 3) are natural polyphenolic compounds—flavan-3-ols (or flavanols); which belong to the flavonoid family [26][27]. They can be classified into two groups: free and esterified catechins [27]. The group of non-esterified catechins includes galocatechin, epicatechin, and epigallocatechins, while the group of esterified catechins comprises epigallocatechin gallate, epicatechin gallate, gallocatechin gallate, and catechin gallate [27]. These compounds are present at high concentrations in various fruits, vegetables, and beverages, for example: grapes (Vitis L.), blackberries (Rubus L.), raspberries (Rubus L.), apples (Malus Mill.), pears (Pyrus L.), cherries (Cerasus Mill.), green tea (Camellia sinensis), chocolate, and legumes [28]. Notwithstanding, most studies focus on teas derived from Camellia sinensis when assessing the amount and activity of catechins [29][30]. The amount of catechin in these natural sources is dependent on several factors, among others: variety, location and cultivation practices, season, illumination, and altitude [27]. Despite the fact that these compounds are not crucial in human nutrition, they can support well-being by preventing different ailments [28]. Numerous studies have shown the varying biological activities of these compounds, i.e., antibacterial [31], antifungal [32], antiviral [33], anticancer [34], antimutagenic [35], antiproliferative [36], antioxidative [37], anti-inflammatory [38], UV protection [39], and anti-neurodegenerative [40]. The number of articles on catechin is shown in Figure 4.
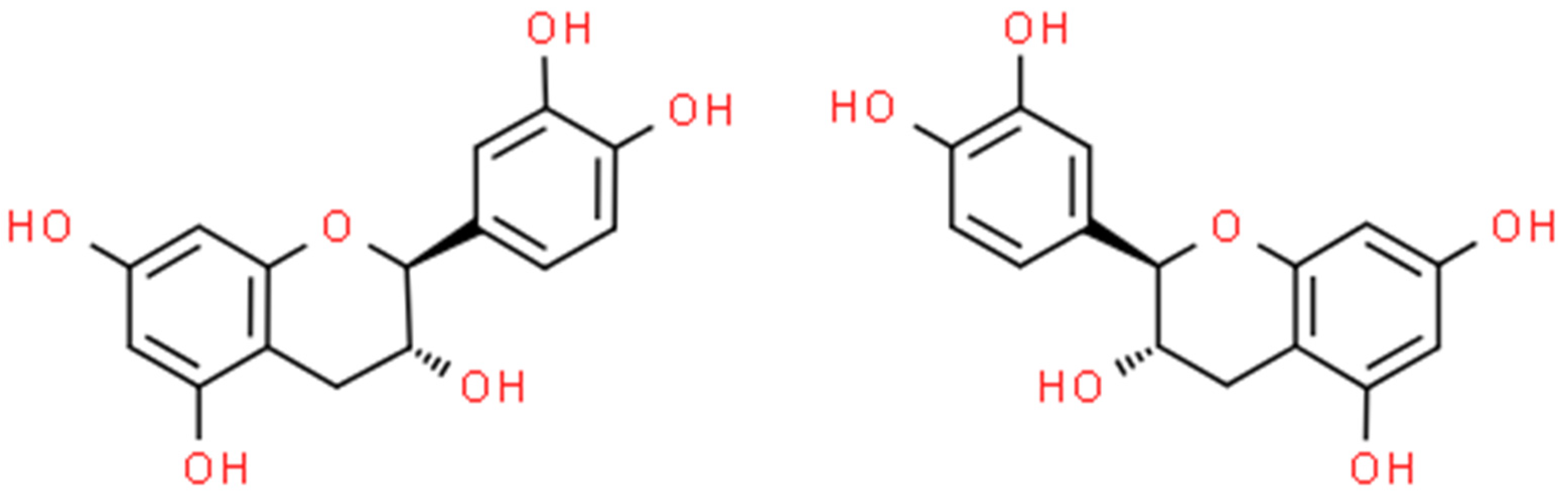
Figure 3. L-(–)-catechin (on the left) and D-(+)-catechin (on the right) structures (ChemSpider database).

Figure 4. The number of papers on catechin per year (PubMed database).
4. Chelerythrine (CHE)
Chelerythrine (Figure 5) is a type III benzophenanthridine alkaloid isolated from four plants of the families Papaveraceae (e.g., Chelidonium majus L., Macleaya cordata (Willd.) R.Br., and Sanguinaria canadensis L.) and Rutaceae (e.g., Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen) [41][42][43]. This bioactive compound possesses anti-inflammatory [44], antibacterial [45], antifungal [46], and antitumor [47] activities. The increase in interest among scientists is clearly visible in Figure 6.
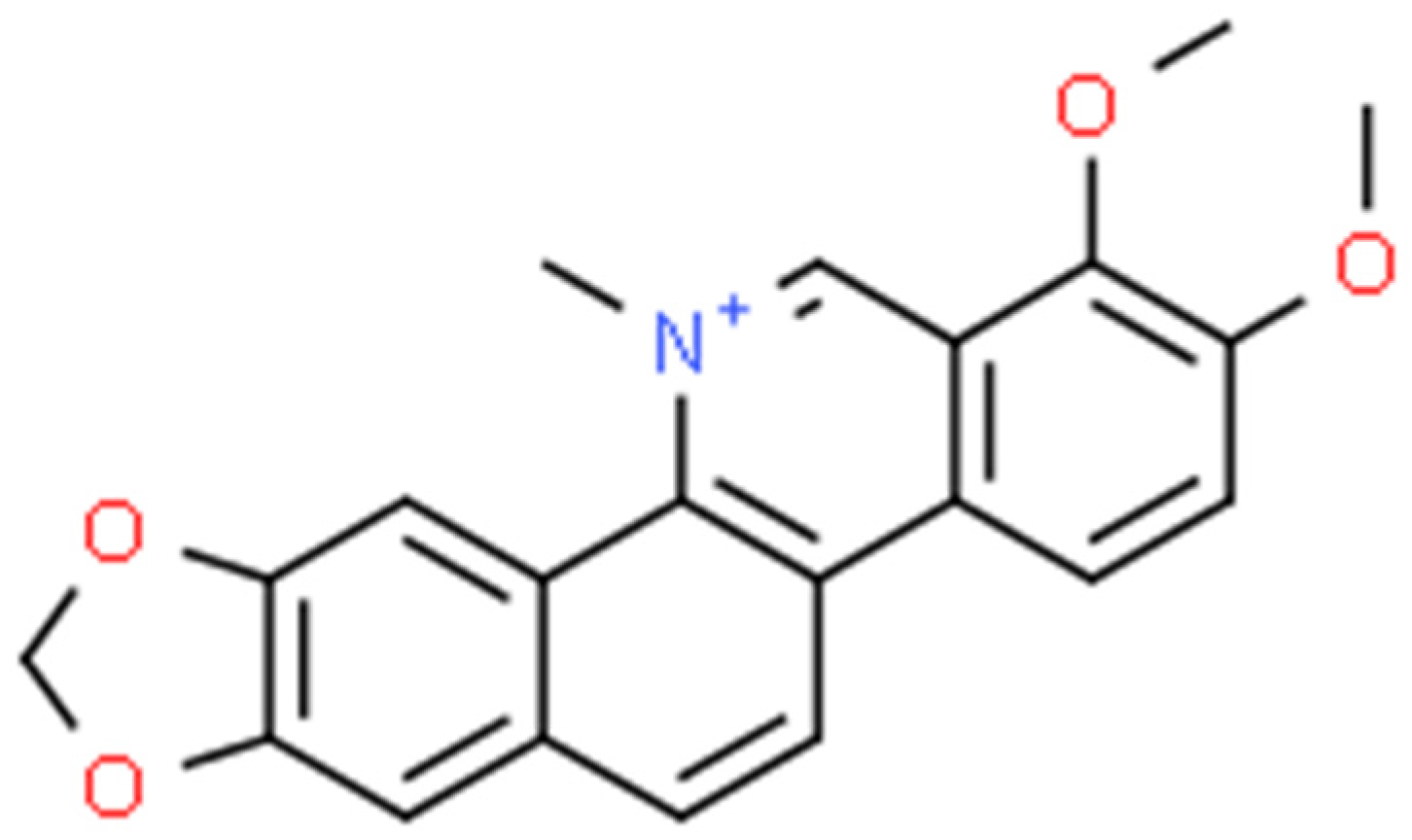
Figure 5. Chelerythrine structure (ChemSpider database).
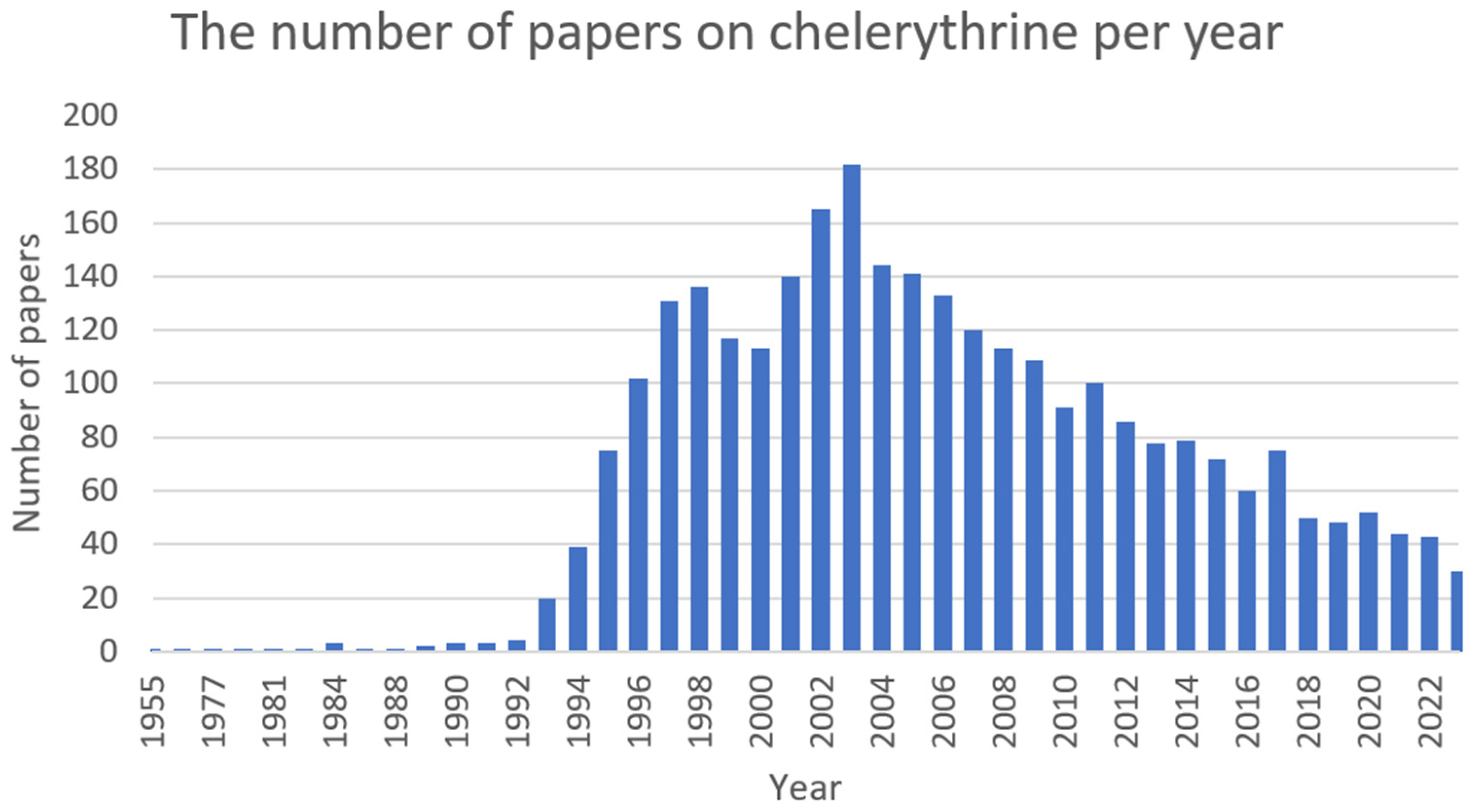
Figure 6. The number of papers on chelerythrine per year (PubMed database).
5. Cinnamaldehyde (CAN)
Cinnamaldehyde (Figure 7) is a bioactive part of cinnamon essential oil and can be extracted from bark, leaves, and twigs of the genus Cinnamomum, which consists of several hundred species [48][49][50][51]. It possesses noticeable health benefits and various applications in the development of pharmacotherapeutic and nutraceutical foods [51]. Cinnamaldehyde has well-documented (Figure 8) activities, including: antimalarial [52], anticancer [53], antifungal [54], antiviral [55], antibacterial [56], anti-diabetes [57], antioxidative, antiperoxidative [58], and anti-inflammation [59].
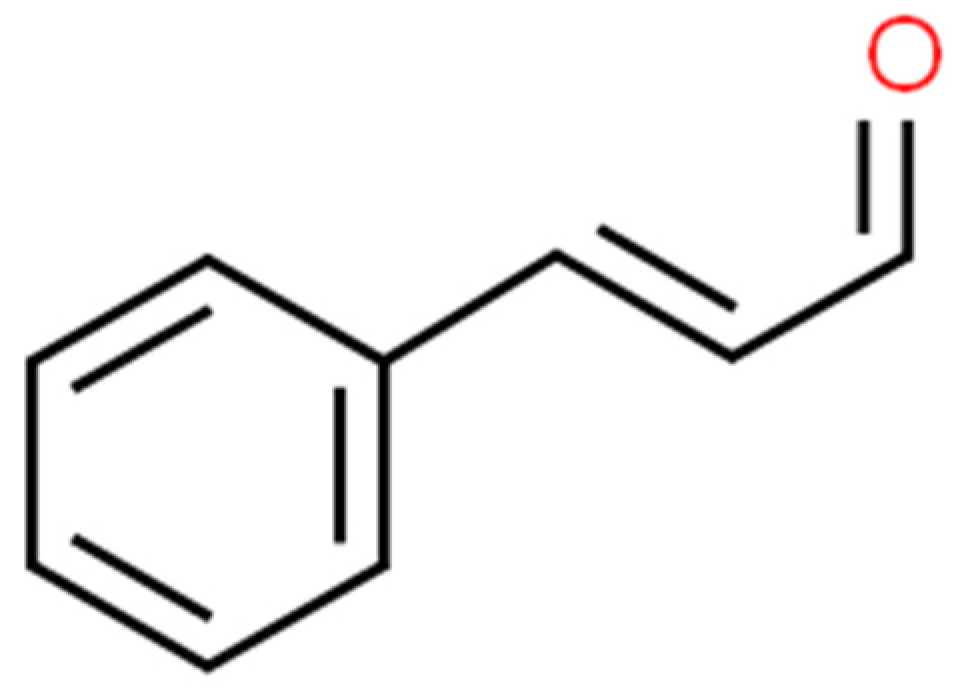
Figure 7. Cinnamaldehyde structure (ChemSpider database).
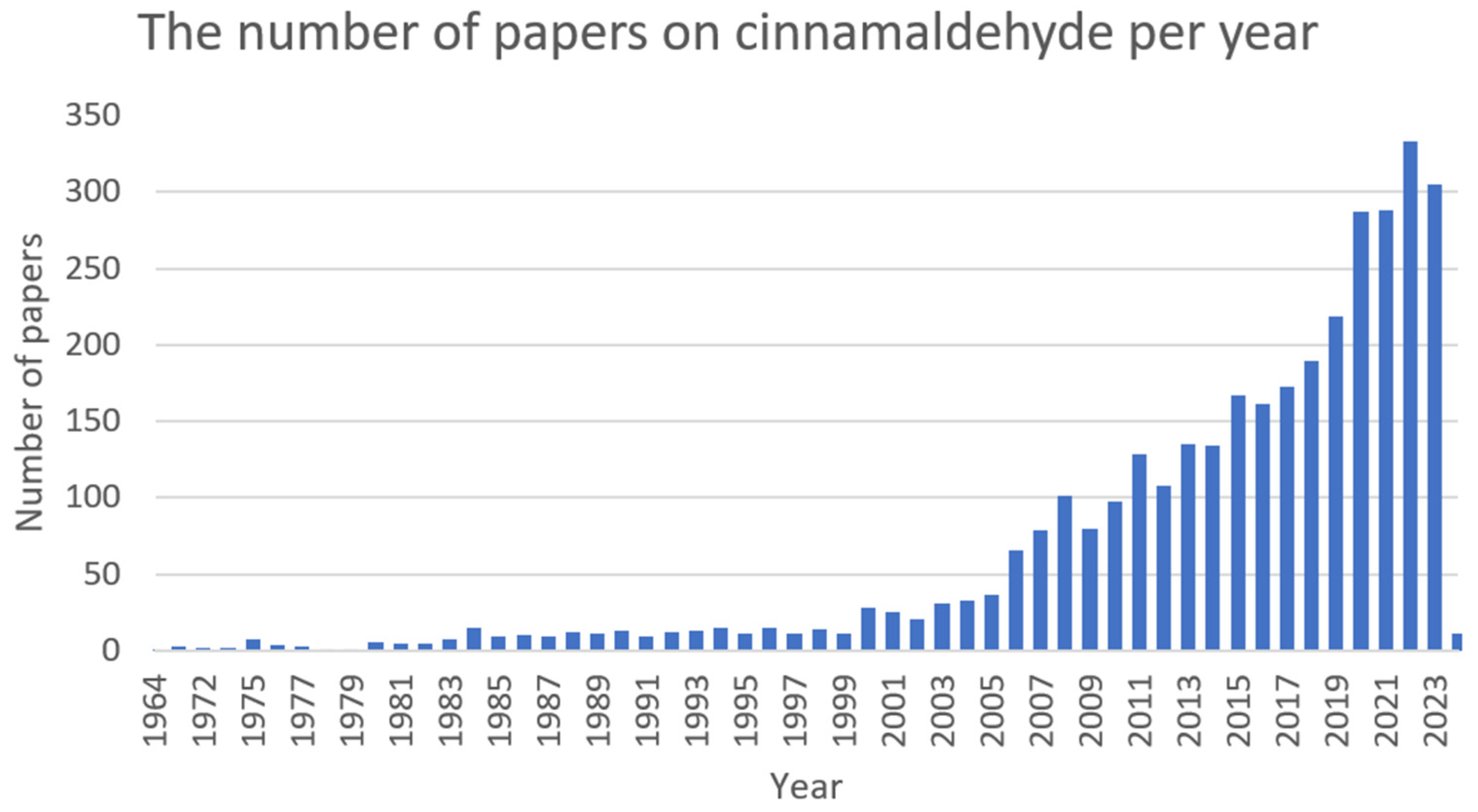
Figure 8. The number of papers on cinnamaldehyde per year (PubMed database).
6. Ellagic Acid (EA)
Ellagic acid (Figure 9) is a bioactive polyphenolic compound that can be extracted from numerous natural sources, such as oak bark, valonea, pomegranate (Punica granatum L.), divi-divi (Caesalpinia coriaria), myrobalan (Terminalia catappa), and algarrobilla (Prosopis humilis) [60][61][62]. Ellagic acid in plants is predominately found ester-linked to sugars in the composition of hydrolysable tannins called ellagitannins [63]. In recent years, it has been attracting great attention (Figure 10) due to its distinct antioxidant [64], anti-inflammatory [65], antifibrotic [66], antimutagenic [67], antiproliferative [68], neuroprotective [69], hepatoprotective [70], cardioprotective [71], wound-healing [72], osteogenic [73], antimicrobial [74], antiviral [75], and antiparasitic [76] activities.
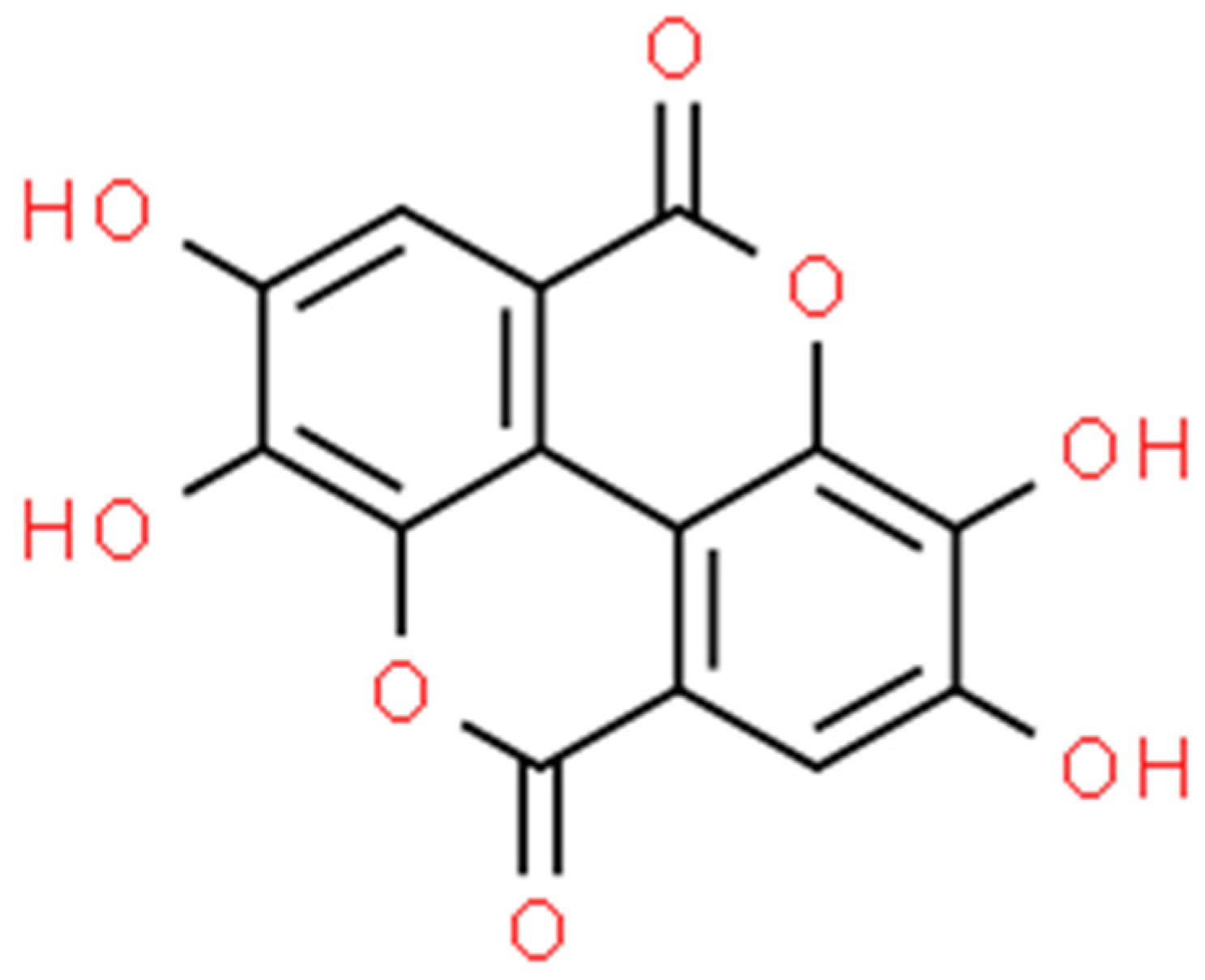
Figure 9. Ellagic acid structure (ChemSpider database).
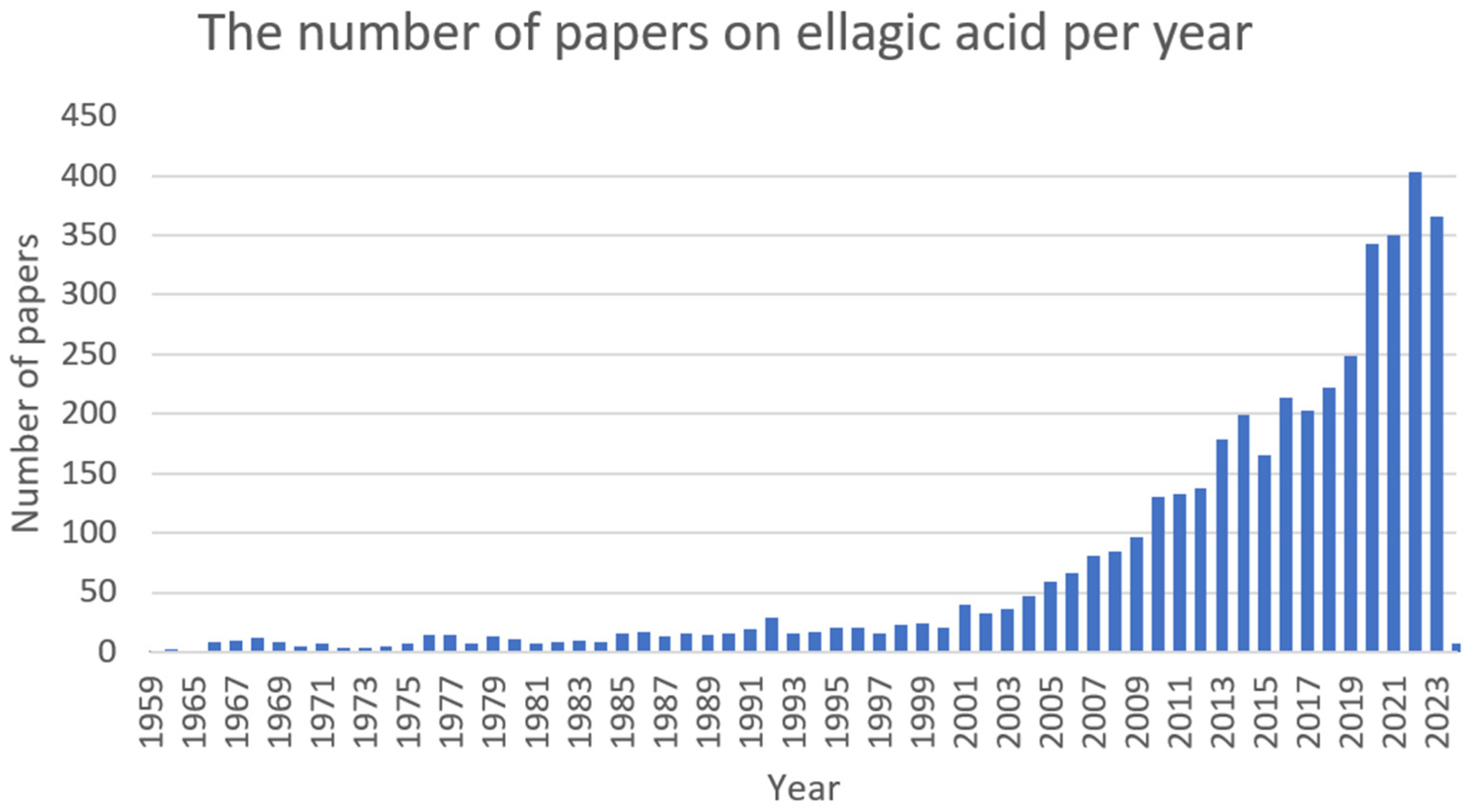
Figure 10. The number of papers on ellagic acid per year (PubMed database).
7. Proanthocyanidin (PAC)
Proanthocyanidins (Figure 11) are condensed tannins obtained by the polymerisation of flavan-3-ol as a structural unit via a C–C bond. On the basis of the degree of polymerisation, they can be divided into oligomeric and polymeric forms. Proanthocyanidins are the most abundant in fruits but can also be found in vegetables and coloured grains. Among the sources with a higher concentration of these compounds are grape seeds (Vitis L.), cranberries (Vaccinium L.), Chinese wolfberries (Lycium chinense), red goji berries (Lycium barbarum), pomegranates (Punica granatum), and red rice (Oryza L.) [77][78][79]. Presumably, they represent the second most abundant class of polyphenols in the plant kingdom after lignin [80]. Numerous in vitro and in vivo studies have confirmed the beneficial human and animal health properties of proanthocyanidins. They possess anti-inflammatory [81], antioxidant [82], antidiabetic [83], neuroprotective [84], cardioprotective [85], antimicrobial [86], immune regulatory [87][88], anti-obesity [89], hypoglycemic [90], hypolipidemic [91], and anticancer [92] activities (Figure 12).

Figure 11. Proanthocyanidin structure (ChemSpider database).
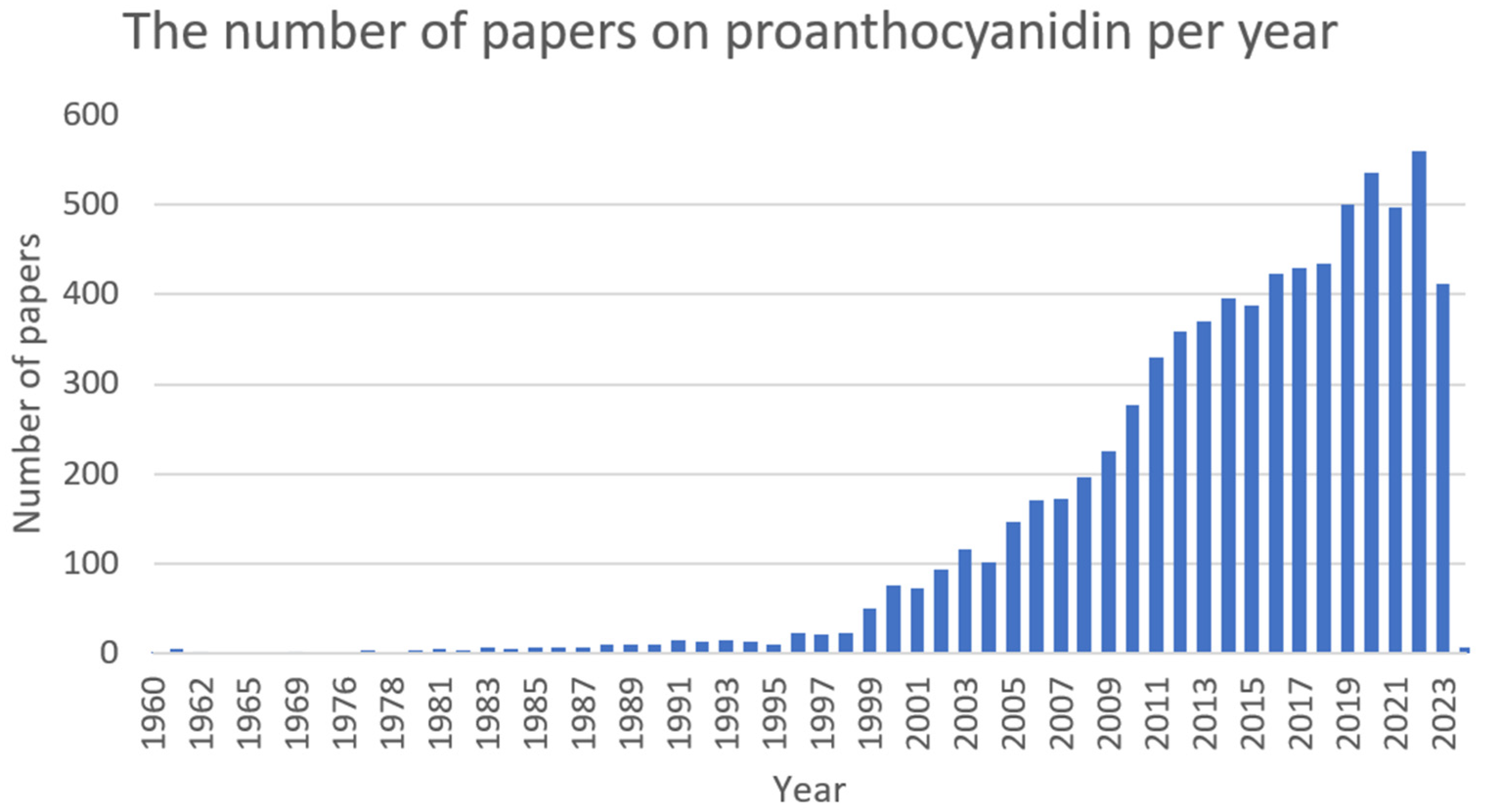
Figure 12. The number of papers on proanthocyanidin per year (PubMed database).
8. Sanguinarine (SG)
Sanguinarine (Figure 13), found in plants such as Sanguinaria canadensis (blood root), Poppy fumaria, Bocconia frutescens, Chelidonium majus, and Macleya cordata [93], is the most commonly used benzophenanthridine alkaloid. It is a benzophenanthridine structural homolog of chelerythrine [94].

Figure 13. Sanguinarine structure (ChemSpider database).
It exhibits antimicrobial [95], antioxidant [96], anti-inflammatory [97], cytotoxic [98], cytostatic [99], cardiac [100], and antiplatelet [101] activity (Figure 14).

Figure 14. The number of papers on sanguinarine per year (PubMed database).
References
- Ohshima, H.; Tatemichi, M.; Sawa, T. Chemical Basis of Inflammation-Induced Carcinogenesis. Arch. Biochem. Biophys. 2003, 417, 3–11.
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218.
- Sans, M.; Panes, J.; Ardite, E.; Elizalde, J.I.; Arce, Y.; Elena, M.; Palacin, A.; Fernandez-Checa, J.C.; Anderson, D.C.; Lobb, R.; et al. VCAM-1 and ICAM-1 Mediate Leukocyte-Endothelial Cell Adhesion in Rat Experimental Colitis. Gastroenterology 1999, 116, 874–883.
- Singh, V.; Kaur, R.; Kumari, P.; Pasricha, C.; Singh, R. ICAM-1 and VCAM-1: Gatekeepers in Various Inflammatory and Cardiovascular Disorders. Clin. Chim. Acta 2023, 548, 117487.
- Abdulkhaleq, L.A.; Assi, M.A.; Abdullah, R.; Zamri-Saad, M.; Taufiq-Yap, Y.H.; Hezmee, M.N.M. The Crucial Roles of Inflammatory Mediators in Inflammation: A Review. Vet. World 2018, 11, 627–635.
- Urban-Chmiel, R.; Marek, A.; Stępień-Pyśniak, D.; Wieczorek, K.; Dec, M.; Nowaczek, A.; Osek, J. Antibiotic Resistance in Bacteria—A Review. Antibiotics 2022, 11, 1079.
- Jian, Z.; Zeng, L.; Xu, T.; Sun, S.; Yan, S.; Yang, L.; Huang, Y.; Jia, J.; Dou, T. Antibiotic Resistance Genes in Bacteria: Occurrence, Spread, and Control. J. Basic Microbiol. 2021, 61, 1049–1070.
- Och, A.; Podgórski, R.; Nowak, R. Biological Activity of Berberine—A Summary Update. Toxins 2020, 12, 713.
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral Activity of Berberine. Arch. Virol. 2020, 165, 1935–1945.
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557.
- Xu, J.; Long, Y.; Ni, L.; Yuan, X.; Yu, N.; Wu, R.; Tao, J.; Zhang, Y. Anticancer Effect of Berberine Based on Experimental Animal Models of Various Cancers: A Systematic Review and Meta-Analysis. BMC Cancer 2019, 19, 589.
- Tan, W.; Li, Y.; Chen, M.; Wang, Y. Berberine Hydrochloride: Anticancer Activity and Nanoparticulate Delivery System. Int. J. Nanomed. 2011, 6, 1773–1777.
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H.; et al. Berberine Is a Novel Cholesterol-Lowering Drug Working through a Unique Mechanism Distinct from Statins. Nat. Med. 2004, 10, 1344–1351.
- Cicero, A.F.G.; Tartagni, E. Antidiabetic Properties of Berberine: From Cellular Pharmacology to Clinical Effects. Hosp. Pract. 2012, 40, 56–63.
- Ilyas, Z.; Perna, S.; Al-thawadi, S.; Alalwan, T.A.; Riva, A.; Petrangolini, G.; Gasparri, C.; Infantino, V.; Peroni, G.; Rondanelli, M. The Effect of Berberine on Weight Loss in Order to Prevent Obesity: A Systematic Review. Biomed. Pharmacother. 2020, 127, 110137.
- Yi, M.; Jun-ping, C.; Li, X.; Xia, L.; Dong-mei, L.; Hui-ping, S. Hypolipidemic Effect of Combination of Berberine and Metformin on Experimental Hyperlipidemia Models. Lab. Anim. Comp. Med. 2014, 2006, 228–231.
- Zhou, M.; Deng, Y.; Liu, M.; Liao, L.; Dai, X.; Guo, C.; Zhao, X.; He, L.; Peng, C.; Li, Y. The Pharmacological Activity of Berberine, a Review for Liver Protection. Eur. J. Pharmacol. 2021, 890, 173655.
- Suadoni, M.T.; Atherton, I. Berberine for the Treatment of Hypertension: A Systematic Review. Complement. Ther. Clin. Pract. 2021, 42, 101287.
- Kulkarni, S.K.; Dhir, A. On the Mechanism of Antidepressant-like Action of Berberine Chloride. Eur. J. Pharmacol. 2008, 589, 163–172.
- Kuo, C.L.; Chi, C.W.; Liu, T.Y. The Anti-Inflammatory Potential of Berberine In Vitro and In Vivo. Cancer Lett. 2004, 203, 127–137.
- Yu, M.; Jin, X.; Liang, C.; Bu, F.; Pan, D.; He, Q.; Ming, Y.; Little, P.; Du, H.; Liang, S.; et al. Berberine for Diarrhea in Children and Adults: A Systematic Review and Meta-Analysis. Ther. Adv. Gastroenterol. 2020, 13, 1–19.
- Lin, S.; Liang, R.; Zhang, T.; Yuan, Y.; Shen, S.; Ye, H. Microarray Analysis of the Transcriptome of the Escherichia coli (E. coli) Regulated by Cinnamaldehyde (CMA). Food Agric. Immunol. 2017, 28, 500–515.
- Shou, J.W.; Shaw, P.C. Therapeutic Efficacies of Berberine against Neurological Disorders: An Update of Pharmacological Effects and Mechanisms. Cells 2022, 11, 796.
- Jian-Ling, J.; Guo-Qiang, H.; Zhen, M.; Gao, P.-J. Antibacterial Mechanisms of Berberine and Reasons for Little Resistance of Bacteria. Chin. Herb. Med. 2010, 3, 27–35.
- Hayashi, K.; Minoda, K.; Nagaoka, Y.; Hayashi, T.; Uesato, S. Antiviral Activity of Berberine and Related Compounds against Human Cytomegalovirus. Bioorg. Med. Chem. Lett. 2007, 17, 1562–1564.
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965.
- Latos-Brozio, M.; Masek, A. Natural Polymeric Compound Based on High Thermal Stability Catechin from Green Tea. Biomolecules 2020, 10, 1191.
- Gadkari, P.V.; Balaraman, M. Catechins: Sources, Extraction and Encapsulation: A Review. Food Bioprod. Process. 2015, 93, 122–138.
- Pedro, A.C.; Maciel, G.M.; Rampazzo Ribeiro, V.; Haminiuk, C.W.I. Fundamental and Applied Aspects of Catechins from Different Sources: A Review. Int. J. Food Sci. Technol. 2020, 55, 429–442.
- Yilmaz, Y. Novel Uses of Catechins in Foods. Trends Food Sci. Technol. 2006, 17, 64–71.
- Gomes, F.M.S.; da Cunha Xavier, J.; dos Santos, J.F.S.; de Matos, Y.M.L.S.; Tintino, S.R.; de Freitas, T.S.; Coutinho, H.D.M. Evaluation of Antibacterial and Modifying Action of Catechin Antibiotics in Resistant Strains. Microb. Pathog. 2018, 115, 175–178.
- Molina-Hernández, J.B.; Scroccarello, A.; Della Pelle, F.; De Flaviis, R.; Compagnone, D.; Del Carlo, M.; Paparella, A.; Chaves Lόpez, C. Synergistic Antifungal Activity of Catechin and Silver Nanoparticles on Aspergillus Niger Isolated from Coffee Seeds. LWT 2022, 169.
- Xu, J.; Xu, Z.; Zheng, W. A Review of the Antiviral Role of Green Tea Catechins. Molecules 2017, 22, 1337.
- Yu, Y.; Deng, Y.; Lu, B.M.; Liu, Y.X.; Li, J.; Bao, J.K. Green Tea Catechins: A Fresh Flavor to Anticancer Therapy. Apoptosis 2014, 19, 1–18.
- Geetha, T.; Garg, A.; Chopra, K.; Pal Kaur, I. Delineation of Antimutagenic Activity of Catechin, Epicatechin and Green Tea Extract. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2004, 556, 65–74.
- Delgado, L.; Fernandes, I.; González-Manzano, S.; De Freitas, V.; Mateus, N.; Santos-Buelga, C. Anti-Proliferative Effects of Quercetin and Catechin Metabolites. Food Funct. 2014, 5, 797–803.
- Zanwar, A.A.; Badole, S.L.; Shende, P.S.; Hegde, M.V.; Bodhankar, S.L. Antioxidant Role of Catechin in Health and Disease; Elsevier Inc.: Amsterdam, The Netherlands, 2013; Volume 1, ISBN 9780123984562.
- Nakanishi, T.; Mukai, K.; Yumoto, H.; Hirao, K.; Hosokawa, Y.; Matsuo, T. Anti-Inflammatory Effect of Catechin on Cultured Human Dental Pulp Cells Affected by Bacteria-Derived Factors. Eur. J. Oral Sci. 2010, 118, 145–150.
- Yoshino, S.; Mitoma, T.; Tsuruta, K.; Todo, H.; Sugibayashi, K. Effect of Emulsification on the Skin Permeation and UV Protection of Catechin. Pharm. Dev. Technol. 2014, 19, 395–400.
- Pervin, M.; Unno, K.; Ohishi, T.; Tanabe, H.; Miyoshi, N.; Nakamura, Y. Beneficial Effects of Green Tea Catechins on Neurodegenerative Diseases. Molecules 2018, 23, 1297.
- Etsè, K.D.; Aïdam, A.V.; Melin, C.; Blanc, N.; Oudin, A.; Courdavault, V.; Creche, J.; Lanoue, A. Optimized Genetic Transformation of Zanthoxylum Zanthoxyloides by Agrobacterium Rhizogenes and the Production of Chelerythrine and Skimmiamine in Hairy Root Cultures. Eng. Life Sci. 2014, 14, 95–99.
- Heng, W.S.; Cheah, S.-C. Chelerythrine Chloride Downregulates β-Catenin and Inhibits Stem Cell Properties of Non-Small Cell Lung Carcinoma. Molecules 2020, 25, 224.
- Chen, N.; Qi, Y.; Ma, X.; Xiao, X.; Liu, Q.; Xia, T.; Xiang, J.; Zeng, J.; Tang, J. Rediscovery of Traditional Plant Medicine: An Underestimated Anticancer Drug of Chelerythrine. Front. Pharmacol. 2022, 13, 906301.
- Zhang, X.; Sun, X.; Wu, J.; Wu, Y.; Wang, Y.; Hu, X.; Wang, X. Berberine Damages the Cell Surface of Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 621.
- He, N.; Wang, P.; Wang, P.; Ma, C.; Kang, W. Antibacterial Mechanism of Chelerythrine Isolated from Root of Toddalia Asiatica (Linn) Lam. BMC Complement. Altern. Med. 2018, 18, 261.
- Yang, X.J.; Miao, F.; Yao, Y.; Cao, F.J.; Yang, R.; Ma, Y.N.; Qin, B.F.; Zhou, L. In Vitro Antifungal Activity of Sanguinarine and Chelerythrine Derivatives against Phytopathogenic Fungi. Molecules 2012, 17, 13026–13035.
- Zhu, Y.; Pan, Y.; Zhang, G.; Wu, Y.; Zhong, W.; Chu, C.; Qian, Y.; Zhu, G. Chelerythrine Inhibits Human Hepatocellular Carcinoma Metastasis In Vitro. Biol. Pharm. Bull. 2018, 41, 36–46.
- Friedman, M. Chemistry, Antimicrobial Mechanisms, and Antibiotic Activities of Cinnamaldehyde against Pathogenic Bacteria in Animal Feeds and Human Foods. J. Agric. Food Chem. 2017, 65, 10406–10423.
- Doyle, A.A.; Stephens, J.C. A Review of Cinnamaldehyde and Its Derivatives as Antibacterial Agents. Fitoterapia 2019, 139, 104405.
- Thirapanmethee, K.; Kanathum, P.; Khuntayaporn, P.; Huayhongthong, S.; Surassmo, S.; Chomnawang, M.T. Cinnamaldehyde: A Plant-Derived Antimicrobial for Overcoming Multidrug-Resistant Acinetobacter Baumannii Infection. Eur. J. Integr. Med. 2021, 48, 101376.
- Ibi, A.A.; Kyuka, C.K. Sources, Extraction and Biological Activities of Cinnamaldehyde. Trends Pharm. Sci. 2022, 2022, 263–282.
- Arianie, L.; Eko Hermanto, F.; Dhiaul Iftitah, E.; Warsito, W.; Widodo, N. Novel Antimalarial Drug Screening Based on Methyl Eugenol, Cinnamaldehyde, and Thiosemicarbazone with Cysteine Protease Inhibition: In Silico Molecular Docking, Molecular Dynamics, and ADMET Studies. J. Pure Appl. Chem. Res. 2022, 11, 102–112.
- Chang, S.; Qin, D.; Wang, L.; Zhang, M.; Yan, R.; Zhao, C. Preparation of Novel Cinnamaldehyde Derivative–BSA Nanoparticles with High Stability, Good Cell Penetrating Ability, and Promising Anticancer Activity. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126765.
- Sun, Q.; Li, J.; Sun, Y.; Chen, Q.; Zhang, L.; Le, T. The Antifungal Effects of Cinnamaldehyde against Aspergillus Niger and Its Application in Bread Preservation. Food Chem. 2020, 317, 126405.
- Goswami, A.; Rahman, A. Antiviral Activity of (Ε)-Cinnamaldehyde Revisited with Nanoscience Tools. Nat. Preced. 2010, 1–4.
- Firmino, D.F.; Cavalcante, T.T.A.; Gomes, G.A.; Firmino, N.C.S.; Rosa, L.D.; De Carvalho, M.G.; Catunda, F.E.A. Antibacterial and Antibiofilm Activities of Cinnamomum sp. Essential Oil and Cinnamaldehyde: Antimicrobial Activities. Sci. World J. 2018, 2018, 7405736.
- Subash Babu, P.; Prabuseenivasan, S.; Ignacimuthu, S. Cinnamaldehyde-A Potential Antidiabetic Agent. Phytomedicine 2007, 14, 15–22.
- Subash-Babu, P.; Alshatwi, A.A.; Ignacimuthu, S. Beneficial Antioxidative and Antiperoxidative Effect of Cinnamaldehyde Protect Streptozotocin-Induced Pancreatic β-Cells Damage in Wistar Rats. Biomol. Ther. 2014, 22, 47–54.
- Cheng, W.X.; Zhong, S.; Meng, X.B.; Zheng, N.Y.; Zhang, P.; Wang, Y.; Qin, L.; Wang, X.L. Cinnamaldehyde Inhibits Inflammation of Human Synoviocyte Cells through Regulation of JAK/STAT Pathway and Ameliorates Collagen-Induced Arthritis in Rats. J. Pharmacol. Exp. Ther. 2020, 373, 302–310.
- Aguilera-Carbo, A.F.; Augur, C.; Prado-Barragan, L.A.; Aguilar, C.N.; Favela-Torres, E. Extraction and Analysis of Ellagic Acid from Novel Complex Sources. Chem. Pap. 2008, 62, 440–444.
- Sepúlveda, L.; Ascacio, A.; Rodríguez-Herrera, R.; Aguilera-Carbó, A.; Aguilar, C.N. Ellagic Acid: Biological Properties and Biotechnological Development for Production Processes. Afr. J. Biotechnol. 2011, 10, 4518–4523.
- Sharifi-Rad, J.; Quispe, C.; Castillo, C.M.S.; Caroca, R.; Lazo-Vélez, M.A.; Antonyak, H.; Polishchuk, A.; Lysiuk, R.; Oliinyk, P.; De Masi, L.; et al. Ellagic Acid: A Review on Its Natural Sources, Chemical Stability, and Therapeutic Potential. Oxid. Med. Cell. Longev. 2022, 2022, 3848084.
- Evtyugin, D.D.; Magina, S.; Evtuguin, D.V. Recent Advances in the Production and Applications of Ellagic Acid and Its Derivatives. A Review. Molecules 2020, 25, 2745.
- Priyadarsini, K.I.; Khopde, S.M.; Kumar, S.S.; Mohan, H. Free Radical Studies of Ellagic Acid, a Natural Phenolic Antioxidant. J. Agric. Food Chem. 2002, 50, 2200–2206.
- Corbett, S.; Daniel, J.; Drayton, R.; Field, M.; Steinhardt, R.; Garrett, N. Evaluation of the Anti-Inflammatory Effects of Ellagic Acid. J. Perianesthesia Nurs. 2010, 25, 214–220.
- Liu, X.; Gao, X.; Li, H.; Li, Z.; Wang, X.; Zhang, L.; Wang, B.; Chen, X.; Meng, X.; Yu, J. Ellagic Acid Exerts Anti-Fibrotic Effects on Hypertrophic Scar Fibroblasts via Inhibition of TGF-Β1/Smad2/3 Pathway. Appl. Biol. Chem. 2021, 64, 67.
- Zahin, M.; Ahmad, I.; Gupta, R.C.; Aqil, F. Punicalagin and Ellagic Acid Demonstrate Antimutagenic Activity and Inhibition of BenzoPyrene Induced DNA Adducts. BioMed Res. Int. 2014, 2014, 467465.
- Kim, J.Y.; Choi, Y.J.; Kim, H.J. Determining the Effect of Ellagic Acid on the Proliferation and Migration of Pancreatic Cancer Cell Lines. Transl. Cancer Res. 2021, 10, 424–433.
- Goudarzi, M.; Mombeini, M.A.; Fatemi, I.; Aminzadeh, A.; Kalantari, H.; Nesari, A.; Najafzadehvarzi, H.; Mehrzadi, S. Neuroprotective Effects of Ellagic Acid against Acrylamide-Induced Neurotoxicity in Rats. Neurol. Res. 2019, 41, 419–428.
- Zhao, L.; Mehmood, A.; Soliman, M.M.; Iftikhar, A.; Iftikhar, M.; Aboelenin, S.M.; Wang, C. Protective Effects of Ellagic Acid against Alcoholic Liver Disease in Mice. Front. Nutr. 2021, 8, 4–6.
- Altamimi, J.Z.; Alfaris, N.A.; Alshammari, G.M.; Alagal, R.I.; Aljabryn, D.H.; Aldera, H.; Alkhateeb, M.A.; Yahya, M.A. Ellagic Acid Protects against Diabetic Cardiomyopathy in Rats by Stimulating Cardiac Silent Information Regulator 1 Signaling. J. Physiol. Pharmacol. 2020, 71, 891–904.
- Lukiswanto, B.S.; Miranti, A.; Sudjarwo, S.A.; Primarizky, H.; Yuniarti, W.M. Evaluation of Wound Healing Potential of Pomegranate (Punica Granatum) Whole Fruit Extract on Skin Burn Wound in Rats (Rattus Norvegicus). J. Adv. Vet. Anim. Res. 2019, 6, 202–207.
- Wardhana, A.S.; Nirwana, I.; Budi, H.S.; Surboyo, M.D.C. Role of Hydroxyapatite and Ellagic Acid in the Osteogenesis. Eur. J. Dent. 2021, 15, 8–12.
- Savic, I.M.; Jocic, E.; Nikolic, V.D.; Popsavin, M.M.; Rakic, S.J.; Savic-Gajic, I.M. The Effect of Complexation with Cyclodextrins on the Antioxidant and Antimicrobial Activity of Ellagic Acid. Pharm. Dev. Technol. 2019, 24, 410–418.
- Acquadro, S.; Civra, A.; Cagliero, C.; Marengo, A.; Rittà, M.; Francese, R.; Sanna, C.; Bertea, C.; Sgorbini, B.; Lembo, D.; et al. Punica Granatum Leaf Ethanolic Extract and Ellagic Acid as Inhibitors of Zika Virus Infection. Planta Med. 2020, 86, 1363–1374.
- Beshbishy, A.M.; Batiha, G.E.S.; Yokoyama, N.; Igarashi, I. Ellagic Acid Microspheres Restrict the Growth of Babesia and Theileria In Vitro and Babesia Microti In Vivo. Parasites Vectors 2019, 12, 269.
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999.
- Dixon, R.A.; Sarnala, S. Proanthocyanidin Biosynthesis—A Matter of Protection. Plant Physiol. 2020, 184, 579–591.
- Qi, Q.; Chu, M.; Yu, X.; Xie, Y.; Li, Y.; Du, Y.; Liu, X.; Zhang, Z.; Shi, J.; Yan, N. Anthocyanins and Proanthocyanidins: Chemical Structures, Food Sources, Bioactivities, and Product Development. Food Rev. Int. 2023, 39, 4581–4609.
- Yu, K.; Song, Y.; Lin, J.; Dixon, R.A. The Complexities of Proanthocyanidin Biosynthesis and Its Regulation in Plants. Plant Commun. 2023, 4, 100498.
- Zhang, J.; Liang, Y.; Ren, L.; Zhang, T. In Vitro Anti-Inflammatory Potency of Sanguinarine and Chelerythrine via Interaction with Glucocorticoid Receptor. eFood 2020, 1, 392–398.
- Zhang, Y.; Dong, Y.; Li, X.; Wang, F. Proanthocyanidin Encapsulated in Ferritin Enhances Its Cellular Absorption and Antioxidant Activity. J. Agric. Food Chem. 2019, 67, 11498–11507.
- Li, X.; Liu, J.; Chang, Q.; Zhou, Z.; Han, R.; Liang, Z. Antioxidant and Antidiabetic Activity of Proanthocyanidins from Fagopyrum Dibotrys. Molecules 2021, 26, 2417.
- Chen, J.; Chen, Y.; Zheng, Y.; Zhao, J.; Yu, H.; Zhu, J.; Li, D. Neuroprotective Effects and Mechanisms of Procyanidins In Vitro and In Vivo. Molecules 2021, 26, 2963.
- Ruan, Y.; Jin, Q.; Zeng, J.; Ren, F.; Xie, Z.; Ji, K.; Wu, L.; Wu, J.; Li, L. Grape Seed Proanthocyanidin Extract Ameliorates Cardiac Remodelling after Myocardial Infarction through PI3K/AKT Pathway in Mice. Front. Pharmacol. 2020, 11, 585984.
- Alfaro-Viquez, E.; Esquivel-Alvarado, D.; Madrigal-Carballo, S.; Krueger, C.G.; Reed, J.D. Antimicrobial Proanthocyanidin-Chitosan Composite Nanoparticles Loaded with Gentamicin. Int. J. Biol. Macromol. 2020, 162, 1500–1508.
- Andersen-Civil, A.I.S.; Arora, P.; Williams, A.R. Regulation of Enteric Infection and Immunity by Dietary Proanthocyanidins. Front. Immunol. 2021, 12, 637603.
- Nawrot-Hadzik, I.; Matkowski, A.; Kubasiewicz-Ross, P.; Hadzik, J. Proanthocyanidins and Flavan-3-Ols in the Prevention and Treatment of Periodontitis—Immunomodulatory Effects, Animal and Clinical Studies. Nutrients 2021, 13, 239.
- Liu, M.; Yun, P.; Hu, Y.; Yang, J.; Khadka, R.B.; Peng, X. Effects of Grape Seed Proanthocyanidin Extract on Obesity. Obes. Facts 2020, 13, 279–291.
- Lu, Z.; Jia, Q.; Wang, R.; Wu, X.; Wu, Y.; Huang, C.; Li, Y. Hypoglycemic Activities of A- and B-Type Procyanidin Oligomer-Rich Extracts from Different Cinnamon Barks. Phytomedicine 2011, 18, 298–302.
- Bladé, C.; Arola, L.; Salvadó, M.J. Hypolipidemic Effects of Proanthocyanidins and Their Underlying Biochemical and Molecular Mechanisms. Mol. Nutr. Food Res. 2010, 54, 37–59.
- Kawahara, S.I.; Ishihara, C.; Matsumoto, K.; Senga, S.; Kawaguchi, K.; Yamamoto, A.; Suwannachot, J.; Hamauzu, Y.; Makabe, H.; Fujii, H. Identification and Characterization of Oligomeric Proanthocyanidins with Significant Anti-Cancer Activity in Adzuki Beans (Vigna Angularis). Heliyon 2019, 5, e02610.
- Mackraj, I.; Govender, T.; Gathiram, P. Sanguinarine. Cardiovasc. Ther. 2008, 26, 75–83.
- Singh, N.; Sharma, B. Toxicological Effects of Berberine and Sanguinarine. Front. Mol. Biosci. 2018, 5, 21.
- Miao, F.; Yang, X.J.; Zhou, L.; Hu, H.J.; Zheng, F.; Ding, X.D.; Sun, D.M.; Zhou, C.D.; Sun, W. Structural Modification of Sanguinarine and Chelerythrine and Their Antibacterial Activity. Nat. Prod. Res. 2011, 25, 863–875.
- Bavarsadi, M.; Mahdavi, A.H.; Ansari-Mahyari, S.; Jahanian, E. Effects of Different Levels of Sanguinarine on Antioxidant Indices, Immunological Responses, Ileal Microbial Counts and Jejunal Morphology of Laying Hens Fed Diets with Different Levels of Crude Protein. J. Anim. Physiol. Anim. Nutr. (Berl). 2017, 101, 936–948.
- Niu, X.; Fan, T.; Li, W.; Xing, W.; Huang, H. The Anti-Inflammatory Effects of Sanguinarine and Its Modulation of Inflammatory Mediators from Peritoneal Macrophages. Eur. J. Pharmacol. 2012, 689, 262–269.
- Serafim, T.L.; Matos, J.A.C.; Sardão, V.A.; Pereira, G.C.; Branco, A.F.; Pereira, S.L.; Parke, D.; Perkins, E.L.; Moreno, A.J.M.; Holy, J.; et al. Sanguinarine Cytotoxicity on Mouse Melanoma K1735-M2 Cells-Nuclear vs. Mitochondrial Effects. Biochem. Pharmacol. 2008, 76, 1459–1475.
- Xu, X.; Deng, L.; Tang, Y.; Li, J.; Zhong, T.; Hao, X.; Fan, Y.; Mu, S. Cytostatic Activity of Sanguinarine and a Cyanide Derivative in Human Erythroleukemia Cells Is Mediated by Suppression of C-MET/MAPK Signaling. Int. J. Mol. Sci. 2023, 24, 8113.
- Chien, M.H.; Yu, W.C.; Jiunn, W.L.; Huei, W.C.; Jaw, J.K. Induction of Contracture and Extracellular Ca2+ Influx in Cardiac Muscle by Sanguinarine: A Study on Cardiotoxicity of Sanguinarine. J. Biomed. Sci. 2005, 12, 399–407.
- Jeng, J.H.; Wu, H.L.; Lin, B.R.; Lan, W.H.; Chang, H.H.; Ho, Y.S.; Lee, P.H.; Wang, Y.J.; Wang, J.S.; Chen, Y.J.; et al. Antiplatelet Effect of Sanguinarine Is Correlated to Calcium Mobilization, Thromboxane and CAMP Production. Atherosclerosis 2007, 191, 250–258.
More
Information
Subjects:
Health Care Sciences & Services
Contributors
MDPI registered users' name will be linked to their SciProfiles pages. To register with us, please refer to https://encyclopedia.pub/register
:
View Times:
541
Revisions:
2 times
(View History)
Update Date:
17 Feb 2024
Notice
You are not a member of the advisory board for this topic. If you want to update advisory board member profile, please contact office@encyclopedia.pub.
OK
Confirm
Only members of the Encyclopedia advisory board for this topic are allowed to note entries. Would you like to become an advisory board member of the Encyclopedia?
Yes
No
${ textCharacter }/${ maxCharacter }
Submit
Cancel
Back
Comments
${ item }
|
More
No more~
There is no comment~
${ textCharacter }/${ maxCharacter }
Submit
Cancel
${ selectedItem.replyTextCharacter }/${ selectedItem.replyMaxCharacter }
Submit
Cancel
Confirm
Are you sure to Delete?
Yes
No




