Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
The rising prevalence of drug-resistant bacteria underscores the need to search for innovative and nature-based solutions. One of the approaches may be the use of plants that constitute a rich source of miscellaneous compounds with a wide range of biological properties. Natural products are one of the most promising and intensively examined agents to combat the consequences of the overuse and misuse of classical antibiotics.
- plants
- bioactive compounds
- antibacterial activity
- biological activity
1. Introduction
Inflammation is a complex disease process caused by endo- or exogenous factors, which can be biological (e.g., bacteria, viruses, fungi, protozoa, endotoxins, exotoxins), physical (e.g., mechanical, ionising radiation, magnetic field, ultrasound waves), or chemical (e.g., acids, bases) [1]. In the process of an inflammatory reaction, cells of the immune system (e.g., macrophages, neutrophils, monocytes, lymphocytes, mast cells), connective tissue cells (e.g., eicosanoids, i.e., prostaglandins, leukotrienes, and lipoxins), certain blood proteins (e.g., complement components, kinins, histamine, serotonin), and blood vessels are involved [2]. Additionally, adhesion molecules, responsible for interactions between leukocytes and endothelial cells, are involved in the pathomechanism of the inflammatory response. These include selectins (P and E-present on the epithelial surface, L-present on the surface of leukocytes), immunoglobulins (ICAM-intercellular, VCAM-vascular), integrins, and mucin-like glycoproteins [3,4]. The expression of these adhesion molecules is dependent on the presence of inflammatory mediators in the environment, i.e., histamine, platelet activating factor (PAF), IL-1, and TNF-α [5]. Recognition of the relevant adhesion molecules initiates the body’s response to the inflammatory agent, which can take the form of (1) cellular (T-lymphocyte involvement)-directed mostly against intracellular pathogens, (2) humoral (B-lymphocyte involvement)-protecting mainly against extracellular microorganisms, and (3) hemostatic (blood component involvement)-leading to platelet aggregation, clot formation, and intravascular coagulation.
Bacterial resistance is related to the adaptation and survival of the microorganism to a new environment in the presence of an antibiotic. Bacteria can: (1) block the penetration of a drug into a bacterial cell (e.g., resistance to β-lactam antibiotics, tetracyclines); (2) prevent the drug from reaching its target site in the cell (e.g., resistance to fluoroquinolones, tetracyclines); (3) modify the drug’s target site (e.g., resistance to β-lactam antibiotics, fluoroquinolones, macrolides, aminoglycosides, glycopeptides); and (4) inactivate or alter the drug by producing special enzymes (e.g., resistance to β-lactam antibiotics, aminoglycosides, chloramphenicol) [39]. In most cases, resistance is genetically determined. Spontaneous point mutations and recombination of genetic material can lead to resistant strains. In addition, this material can be transferred between microorganisms by (1) transformation (acquisition and incorporation of a free DNA fragment into the genome), (2) transduction (introduction of resistance genes by a bacteriophage), and (3) conjugation (transfer of plasmid-extra-chromosomal genetic material and transposons-fragments of DNA capable of movement) [40]. In these ways, resistance genes, not only between pathogenic bacteria but also their reservoirs, can become representatives of the microbiome.
2. Berberine (BRB)
Berberine (Figure 1) is a plant metabolite that belongs to the group of isoquinoline alkaloids and exhibits low toxicity as well as strong biological and pharmacological activities [44,45]. It is a very important natural alkaloid that is used to synthesise various bioactive derivatives by modifying functional groups that are important for the development of new and selective treatments [46]. Among the Berberidaceae family, the genus Berberis includes approximately 450–500 species, which are the major natural sources of berberine [45,46]. This active compound can be found in the leaves, stems, twigs, barks, rhizomes, and roots of many medicinal plants [46]. At present, berberine is of great interest due to its, among others, anticancer [47,48], cholesterol-lowering [49], antidiabetic [50], anti-obesity [51], hypolipidemic [52], hepatoprotective [53], antihypertensive [54], antidepressant [55], anti-inflammatory [56], antidiarrheal [57], anti-neurological [58,59], antibacterial [60], and antiviral [61] properties. However, the favourable applications of berberine are restricted by the weak pharmacokinetics of the compound. Nonetheless, berberine appears to be very effective and can be used in combination with other chemotherapeutics or treatments [44]. The growing interest in this compound can be seen in the increasing number of articles (Figure 2).
Figure 1. Berberine structure (ChemSpider database).
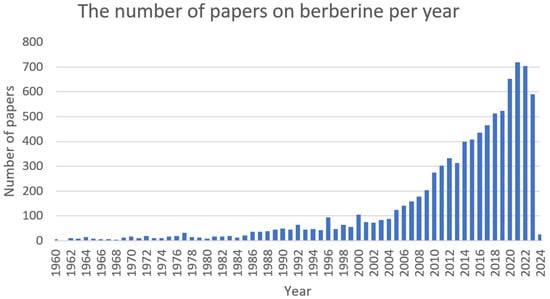
Figure 2. The number of papers on berberine per year (PubMed database).
3. Catechin (CT)
Catechins (Figure 3) are natural polyphenolic compounds—flavan-3-ols (or flavanols); which belong to the flavonoid family [106,107]. They can be classified into two groups: free and esterified catechins [107]. The group of non-esterified catechins includes galocatechin, epicatechin, and epigallocatechins, while the group of esterified catechins comprises epigallocatechin gallate, epicatechin gallate, gallocatechin gallate, and catechin gallate [107]. These compounds are present at high concentrations in various fruits, vegetables, and beverages, for example: grapes (Vitis L.), blackberries (Rubus L.), raspberries (Rubus L.), apples (Malus Mill.), pears (Pyrus L.), cherries (Cerasus Mill.), green tea (Camellia sinensis), chocolate, and legumes [108]. Notwithstanding, most studies focus on teas derived from Camellia sinensis when assessing the amount and activity of catechins [109,110]. The amount of catechin in these natural sources is dependent on several factors, among others: variety, location and cultivation practices, season, illumination, and altitude [107]. Despite the fact that these compounds are not crucial in human nutrition, they can support well-being by preventing different ailments [108]. Numerous studies have shown the varying biological activities of these compounds, i.e., antibacterial [111], antifungal [112], antiviral [113], anticancer [114], antimutagenic [115], antiproliferative [116], antioxidative [117], anti-inflammatory [118], UV protection [119], and anti-neurodegenerative [120]. The number of articles on catechin is shown in Figure 4.
Figure 3. L-(–)-catechin (on the left) and D-(+)-catechin (on the right) structures (ChemSpider database).
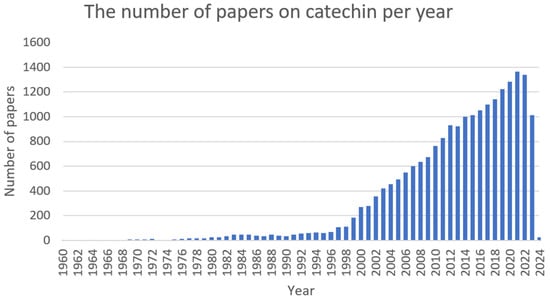
Figure 4. The number of papers on catechin per year (PubMed database).
4. Chelerythrine (CHE)
Chelerythrine (Figure 5) is a type III benzophenanthridine alkaloid isolated from four plants of the families Papaveraceae (e.g., Chelidonium majus L., Macleaya cordata (Willd.) R.Br., and Sanguinaria canadensis L.) and Rutaceae (e.g., Zanthoxylum asiaticum (L.) Appelhans, Groppo & J.Wen) [145,146,147]. This bioactive compound possesses anti-inflammatory [71], antibacterial [148], antifungal [149], and antitumor [150] activities. The increase in interest among scientists is clearly visible in Figure 6.
Figure 5. Chelerythrine structure (ChemSpider database).
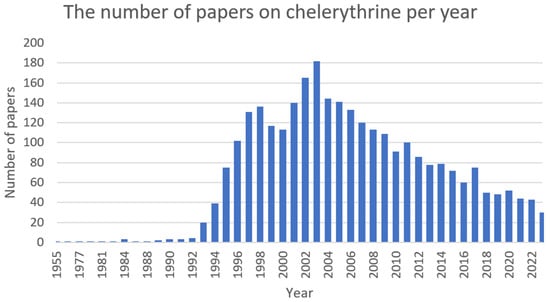
Figure 6. The number of papers on chelerythrine per year (PubMed database).
5. Cinnamaldehyde (CAN)
Cinnamaldehyde (Figure 7) is a bioactive part of cinnamon essential oil and can be extracted from bark, leaves, and twigs of the genus Cinnamomum, which consists of several hundred species [156,157,158,159]. It possesses noticeable health benefits and various applications in the development of pharmacotherapeutic and nutraceutical foods [159]. Cinnamaldehyde has well-documented (Figure 8) activities, including: antimalarial [160], anticancer [161], antifungal [162], antiviral [163], antibacterial [164], anti-diabetes [165], antioxidative, antiperoxidative [166], and anti-inflammation [167].
Figure 7. Cinnamaldehyde structure (ChemSpider database).
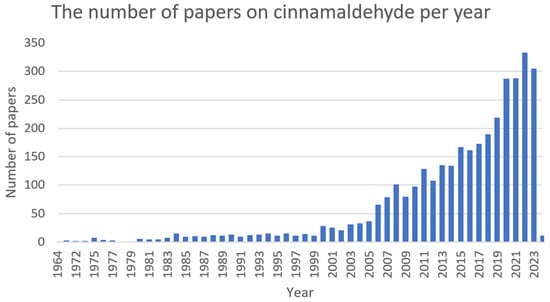
Figure 8. The number of papers on cinnamaldehyde per year (PubMed database).
6. Ellagic Acid (EA)
Ellagic acid (Figure 9) is a bioactive polyphenolic compound that can be extracted from numerous natural sources, such as oak bark, valonea, pomegranate (Punica granatum L.), divi-divi (Caesalpinia coriaria), myrobalan (Terminalia catappa), and algarrobilla (Prosopis humilis) [216,217,218]. Ellagic acid in plants is predominately found ester-linked to sugars in the composition of hydrolysable tannins called ellagitannins [219]. In recent years, it has been attracting great attention (Figure 10) due to its distinct antioxidant [220], anti-inflammatory [221], antifibrotic [222], antimutagenic [223], antiproliferative [224], neuroprotective [225], hepatoprotective [226], cardioprotective [227], wound-healing [228], osteogenic [229], antimicrobial [230], antiviral [231], and antiparasitic [232] activities.
Figure 9. Ellagic acid structure (ChemSpider database).
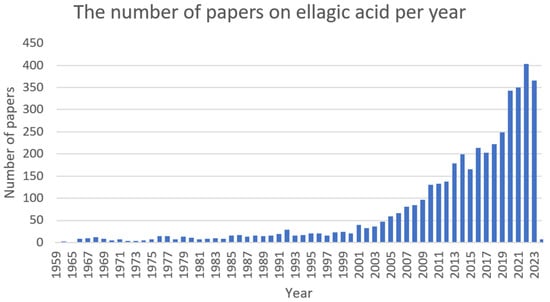
Figure 10. The number of papers on ellagic acid per year (PubMed database).
7. Proanthocyanidin (PAC)
Proanthocyanidins (Figure 11) are condensed tannins obtained by the polymerisation of flavan-3-ol as a structural unit via a C–C bond. On the basis of the degree of polymerisation, they can be divided into oligomeric and polymeric forms. Proanthocyanidins are the most abundant in fruits but can also be found in vegetables and coloured grains. Among the sources with a higher concentration of these compounds are grape seeds (Vitis L.), cranberries (Vaccinium L.), Chinese wolfberries (Lycium chinense), red goji berries (Lycium barbarum), pomegranates (Punica granatum), and red rice (Oryza L.) [252,253,254]. Presumably, they represent the second most abundant class of polyphenols in the plant kingdom after lignin [255]. Numerous in vitro and in vivo studies have confirmed the beneficial human and animal health properties of proanthocyanidins. They possess anti-inflammatory [256], antioxidant [257], antidiabetic [258], neuroprotective [259], cardioprotective [260], antimicrobial [261], immune regulatory [262,263], anti-obesity [264], hypoglycemic [265], hypolipidemic [266], and anticancer [267] activities (Figure 12).
Figure 11. Proanthocyanidin structure (ChemSpider database).
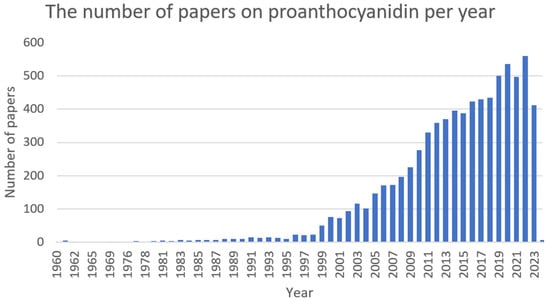
Figure 12. The number of papers on proanthocyanidin per year (PubMed database).
8. Sanguinarine (SG)
Sanguinarine (Figure 13), found in plants such as Sanguinaria canadensis (blood root), Poppy fumaria, Bocconia frutescens, Chelidonium majus, and Macleya cordata [279], is the most commonly used benzophenanthridine alkaloid. It is a benzophenanthridine structural homolog of chelerythrine [280].
Figure 13. Sanguinarine structure (ChemSpider database).
It exhibits antimicrobial [281], antioxidant [282], anti-inflammatory [283], cytotoxic [284], cytostatic [285], cardiac [286], and antiplatelet [287] activity (Figure 14).
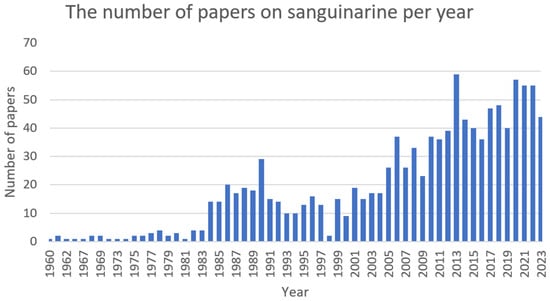
Figure 14. The number of papers on sanguinarine per year (PubMed database).
This entry is adapted from the peer-reviewed paper 10.3390/ijms25042100
This entry is offline, you can click here to edit this entry!
