
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Antoine Guichet | -- | 2820 | 2024-02-09 13:00:31 | | | |
| 2 | Catherine Yang | Meta information modification | 2820 | 2024-02-17 04:34:36 | | |
Video Upload Options
Oogenesis is a developmental process leading to the formation of an oocyte, a haploid gamete, which upon fertilisation and sperm entry allows the male and the female pronuclei to fuse and give rise to a zygote. In addition to forming a haploid gamete, oogenesis builds up a store of proteins, mRNAs, and organelles in the oocyte needed for the development of the future embryo. In several species, such as Drosophila, the polarity axes determinants of the future embryo must be asymmetrically distributed prior to fertilisation. In the Drosophila oocyte, the correct positioning of the nucleus is essential for establishing the dorsoventral polarity axis of the future embryo and allowing the meiotic spindles to be positioned in close vicinity to the unique sperm entry point into the oocyte.
1. Anatomy and Development of the Drosophila Egg Chamber

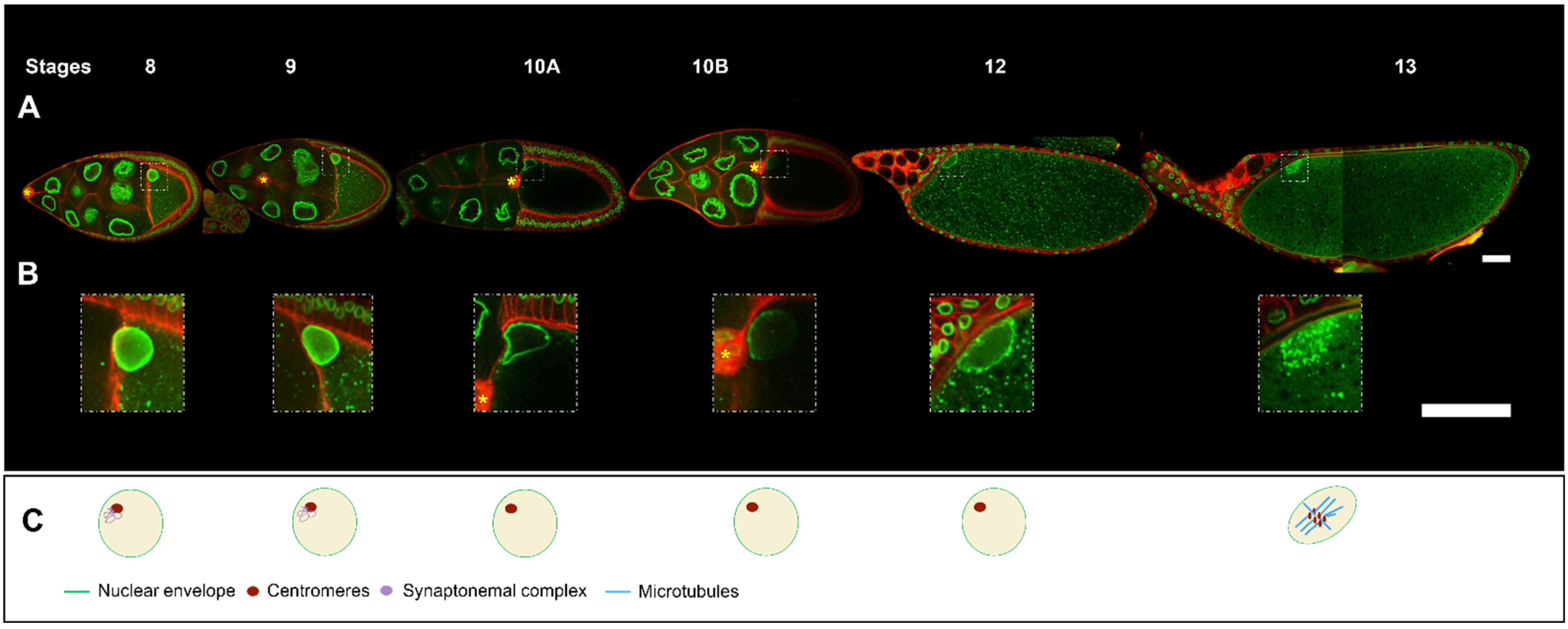
-
During early oogenesis, before stage 6, the grk mRNA is localized close to the nucleus hemisphere facing the posterior of the oocyte, and its translation leads to the activation of the EGFr in about 20 follicle cells adjacent to the oocyte. This induces a differentiation program necessary for building the posterior structures of the eggshell [35][36][37][38] and for the emission, in conjunction with the JAK-STAT signalling pathway, of a signal from those cells, that later triggers a repolarization of the MT network of the oocyte and the start of nucleus migration [37][38][39] (Figure 1C).
-
With the asymmetrical positioning of the nucleus to an antero-dorsal position, the grk mRNA, still associated with the nucleus, relocalises in the vicinity of the antero-dorsal follicle cells where its translation triggers a second wave of EGFr activation in a gradient pattern (Figure 1E). This enables the formation of two groups of follicle cells, which forms the dorsal appendages. Through a series of steps, the cells that lack EGFr activation because they do not receive the Grk signal, secrete the ligand triggering the formation of most of the different germ layers of the embryo via the activation of the Toll receptor [7][8]. A crucial step in this process is the asymmetrical positioning of the nucleus, which functions as a symmetry-breaking event for the formation of the dorsoventral axis of the eggshell and of the future embryo. Because the lateral follicle cells are all equivalent before this second wave of Grk signaling, and because there are no markers that predict where the nucleus would move within the oocyte, it is thought the nucleus can migrate towards any position representing the intersection between the anterior and lateral parts of the oocyte. Further support for this view comes from an elegant experiment showing in oocytes with two nuclei, the nuclei migrate to random positions with respect to each other [40].
2. Steps in Nucleus Positioning during Oocyte Development

Oocyte Nucleus Positioning and Cytoskeleton
References
- King, R.C. Ovarian Development in Drosophila Melanogaster; Academic Press: New York, NY, USA, 1970.
- McLaughlin, J.M.; Bratu, D.P. Drosophila melanogaster Oogenesis: An Overview. In Drosophila Oogenesis: Methods and Protocols; Bratu, D.P., McNeil, G.P., Eds.; Springer New York: New York, NY, USA, 2015; pp. 1–20.
- Almonacid, M.; Terret, M.-E.; Verlhac, M.-H. Control of nucleus positioning in mouse oocytes. Semin. Cell Dev. Biol. 2017, 82, 34–40.
- Hughes, S.E.; Miller, D.E.; Miller, A.L.; Hawley, R.S. Female Meiosis: Synapsis, Recombination, and Segregation in Drosophila melanogaster. Genetics 2018, 208, 875–908.
- Mahowald, A.P.; Tiefert, M. Fine structural changes in the Drosophila oocyte nucleus during a short period of RNA synthesis—An autoradiographic and ultrastructural study of RNA synthesis in the oocyte nucleus of Drosophila. Wilhelm Roux. Arch. Entwickl. Mech. Org. 1970, 165, 8–25.
- Liu, J.L.; Buszczak, M.; Gall, J.G. Nuclear bodies in the Drosophila germinal vesicle. Chromosom. Res. 2006, 14, 465–475.
- Roth, S.; Lynch, J.A. Symmetry breaking during Drosophila oogenesis. Cold Spring Harb. Perspect. Biol. 2009, 1, a001891.
- Moussian, B.; Roth, S. Dorsoventral axis formation in the Drosophila embryo—Shaping and transducing a morphogen gradient. Curr. Biol. 2005, 15, 887–899.
- Duchek, P.; Rørth, P. Guidance of Cell Migration by EGF Receptor Signaling During Drosophila Oogenesis. Science 2001, 291, 131–133.
- Huynh, J.R.; St Johnston, D. The origin of asymmetry: Early polarisation of the Drosophila germline cyst and oocyte. Curr. Biol. 2004, 14, 438–449.
- Adelaide, C. Electron microscopy of meiosis in Drosophila melanogaster females. I. Structure, arrangement, and temporal change of the synaptonemal complex in wild-type. Chromosoma 1975, 51, 157–182.
- Theurkauf, W.E.; Alberts, B.M.; Nung Jan, Y.; Jongens, T.A.; Jan, Y.N.; Jongens, T.A. A central role for microtubules in the differentiation of Drosophila oocytes. Development 1993, 1169–1180.
- Nicolas, E.; Chenouard, N.; Olivo-Marin, J.-C.J.-C.; Guichet, A. A dual role for actin and microtubule cytoskeleton in the transport of golgi units from the nurse cells to the oocyte across ring canals. Mol. Biol. Cell 2009, 20, 556–568.
- Lu, W.; Lakonishok, M.; Gelfand, V.I. Gatekeeper function for Short stop at the ring canals of the Drosophila ovary. Curr. Biol. 2021, 31, 3207–3219.e5.
- Lu, W.; Lakonishok, M.; Serpinskaya, A.S.; Gelfand, V.I. A novel mechanism of bulk cytoplasmic transport by cortical dynein in Drosophila ovary. eLife 2022, 11, e75538.
- Januschke, J.; Gervais, L.; Gillet, L.; Keryer, G.; Bornens, M.; Guichet, A. The centrosome-nucleus complex and microtubule organization in the Drosophila oocyte. Development 2006, 133, 129–139.
- Nashchekin, D.; Fernandes, A.R.; St. Johnston, D. Patronin/Shot Cortical Foci Assemble the Noncentrosomal Microtubule Array that Specifies the Drosophila Anterior-Posterior Axis. Dev. Cell 2016, 38, 61–72.
- Tissot, N.; Lepesant, J.-A.A.; Bernard, F.; Legent, K.; Bosveld, F.; Martin, C.; Faklaris, O.; Bellaïche, Y.; Coppey, M.; Guichet, A. Distinct molecular cues ensure a robust microtubule-dependent nuclear positioning in the Drosophila oocyte. Nat. Commun. 2017, 8, 15168.
- Nashchekin, D.; Busby, L.; Jakobs, M.; Squires, I.; St. Johnston, D. Symmetry breaking in the female germline cyst. Science 2021, 374, 874–879.
- Roper, K.; Brown, N. A Spectraplakin Is Enriched on the Fusome and Organizes Microtubules during Oocyte Specification in Drosophila. Curr. Biol. 2004, 14, 99–110.
- Bolívar, J.; Huynh, J.-R.; López-Schier, H.; González, C.; St. Johnston, D.; González-Reyes, A. Centrosome migration into the Drosophila oocyte is independent of BicD and egl, and of the organisation of the microtubule cytoskeleton. Development 2001, 1909, 1889–1909.
- Zhao, T.; Graham, O.S.; Raposo, A.; St. Johnston, D. Growing Microtubules Push the Oocyte Nucleus to Polarize the Drosophila Dorsal-Ventral Axis. Science 2012, 336, 999–1003.
- Loh, M.; Bernard, F.; Guichet, A. Kinesin-1 promotes centrosome clustering and nuclear migration in the Drosophila oocyte. Development 2023, 150, dev201728.
- Pimenta-Marques, A.; Bento, I.; Lopes, C.A.M.; Duarte, P.; Jana, S.C.; Bettencourt-Dias, M. A mechanism for the elimination of the female gamete centrosome in Drosophila melanogaster. Science 2016, 353, aaf4866.
- St Johnston, D. Polarity and axis formation in the Drosophila female germ line. Curr. Top. Dev. Biol. 2023, 154, 73–97.
- Robinson, D.N.; Cant, K.; Cooley, L. Morphogenesis of Drosophila ovarian ring canals. Development 2015, 2025, 2015–2025.
- Driever, W.; Nüsslein-Volhard, C. A gradient of bicoid protein in Drosophila embryos. Cell 1988, 54, 83–93.
- St. Johnston, D.; Driever, W.; Berleth, T.; Richstein, S.; Nusslein-Volhard, C. Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development 1989, 107, 13–19.
- Ephrussi, A.; Lehmann, R. Induction of germ cell fate by oskar. Nature 1992, 358, 387–392.
- Ephrussi, A.; Dickinson, L.K.; Lehmann, R. Oskar Organizes the Germ Plasm and Directs Localization of the Posterior Determinant Nanos. Cell 1991, 66, 37–50.
- Gavis, E.R.; Lehmann, R. Localization of nanos RNA controls embryonic polarity. Cell 1992, 71, 301–313.
- Kim-Ha, J.; Smith, J.L.; Macdonald, P.M. oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 1991, 66, 23–35.
- Jeske, M.; Müller, C.W.; Ephrussi, A. The LOTUS domain is a conserved DEAD-box RNA helicase regulator essential for the recruitment of Vasa to the germ plasm and nuage. Genes Dev. 2017, 31, 939–952.
- Neuman-Silberberg, F.S.; Schüpbach, T. The drosophila dorsoventral patterning gene gurken produces a dorsally localized RNA and encodes a TGFα-like protein. Cell 1993, 75, 165–174.
- Acaimo, G.-R.; St. Johnston, D. Role of Oocyte Position in Establishment of Anterior-Posterior Polarity in Drosophila. Science 1994, 266, 639–642.
- González-Reyes, A.; St. Johnston, D. Patterning of the follicle cell epithelium along the anterior-posterior axis during Drosophila oogenesis. Development 1998, 125, 2837–2846.
- Roth, S.; Neuman-Silberberg, F.S.; Barcelo, G.; Schüpbach, T. cornichon and the EGF receptor signaling process are necessary for both anterior-posterior and dorsal-ventral pattern formation in Drosophila. Cell 1995, 81, 967–978.
- González-Reyes, A.; Elliott, H.; St. Johnston, D. Polarization of both major body axes in Drosophila by gurken-torpedo signalling. Nature 1995, 375, 654–658.
- Xi, R.; McGregor, J.R.; Harrison, D.A. A gradient of JAK pathway activity patterns the anterior-posterior axis of the follicular epithelium. Dev. Cell 2003, 4, 167–177.
- Roth, S.; Jordan, P.; Karess, R. Binuclear Drosophila oocytes: Consequences and implications for dorsal-ventral patterning in oogenesis and embryogenesis. Development 1999, 126, 927–934.
- Paré, C.; Suter, B. Subcellular localization of Bic-D::GFP is linked to an asymmetric oocyte nucleus. J. Cell Sci. 2000, 113, 2119–2127.
- Guichet, A.; Peri, F.; Roth, S. Stable anterior anchoring of the oocyte nucleus is required to establish dorsoventral polarity of the drosophila egg. Dev. Biol. 2001, 237, 93–106.
- Horne-Badovinac, S. The Drosophila micropyle as a system to study how epithelia build complex extracellular structures. Philos. Trans. R. Soc. B Biol. Sci. 2020, 375, 20190561.
- Koch, E.A.; Spitzer, R.H. Multiple effects of colchicine on oogenesis in Drosophila: Induced sterility and switch of potential oocyte to nurse-cell developmental pathway. Cell Tissue Res. 1983, 228, 21–32.
- Baffet, A.D.D.; Benoit, B.; Januschke, J.; Audo, J.; Gourhand, V.; Roth, S.; Guichet, A. Drosophila tubulin-binding cofactor B is required for microtubule network formation and for cell polarity. Mol. Biol. Cell 2012, 23, 3591–3601.
- Chemla, L.; Guichet, A. Université Paris-Cité, CNRS, Institut Jacques Monod, Paris, France. 2024; manuscript in preparation.
- Manseau, L.; Galley, J.; Phan, H. Profilin is required for posterior patterning of the Drosophila oocyte. Development 1996, 122, 2109–2116.
- Verheyen, E.M.; Cooley, L. Profilin mutations disrupt multiple actin-dependent processes during Drosophila development. Development 1994, 120, 717–728.




