
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Sandra Pascual-García | -- | 1244 | 2024-02-08 10:58:14 | | | |
| 2 | Fanny Huang | -3 word(s) | 1241 | 2024-02-18 09:22:04 | | | | |
| 3 | Fanny Huang | -60 word(s) | 1181 | 2024-02-20 04:20:28 | | |
Video Upload Options
Rheumatoid arthritis (RA) is an autoimmune disease that causes inflammation, pain, and ultimately, bone erosion of the joints. The causes of this disease are multifactorial, including genetic factors, such as the presence of the human leukocyte antigen (HLA)-DRB1*04 variant, alterations in the microbiota, or immune factors including increased cytotoxic T lymphocytes (CTLs), neutrophils, or elevated M1 macrophages which, taken together, produce high levels of pro-inflammatory cytokines. The function exerted by osteoclasts on osteoblasts and other osteoclasts by means of the release of exosomal microRNAs (miRNAs) was focused on. Based on a thorough revision, researchers classified these molecules into three categories according to their function: osteoclast inhibitors (miR-23a, miR-29b, and miR-214), osteoblast inhibitors (miR-22-3p, miR-26a, miR-27a, miR-29a, miR-125b, and miR-146a), and osteoblast enhancers (miR-20a, miR-34a, miR-96, miR-106a, miR-142, miR-199a, miR-324, and miR-486b).
1. Introduction
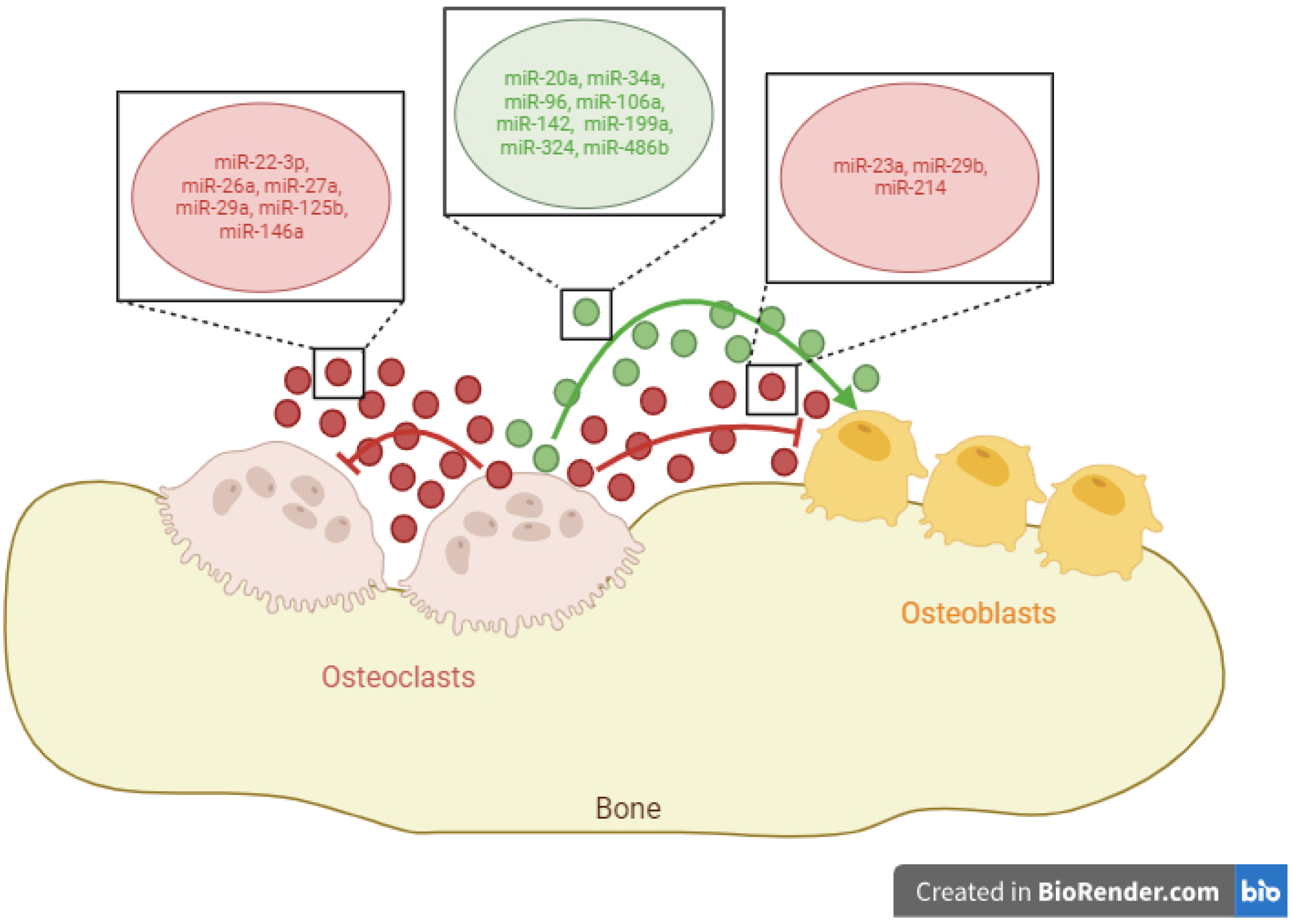
2. Exosomal miRNAs with the Ability to Promote Osteoblast Differentiation

3. Exosomal miRNAs with the Ability to Inhibit Osteoblast Differentiation

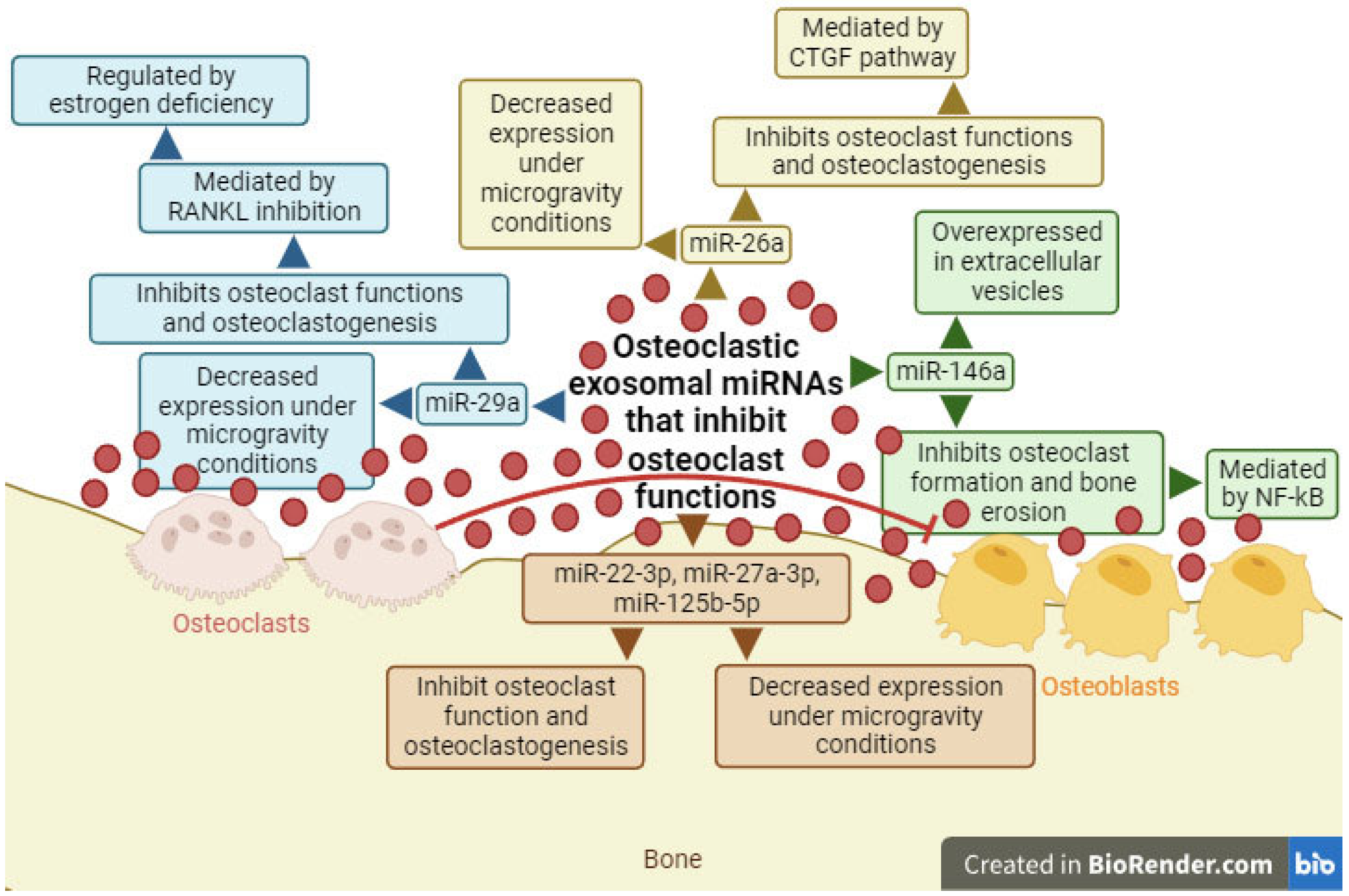
4. Exosomal miRNAs with the Ability to Inhibit Osteoclast Differentiation
References
- Ouboussad, L.; Hunt, L.; Hensor, E.M.A.; Nam, J.L.; Barnes, N.A.; Emery, P.; McDermott, M.F.; Buch, M.H. Profiling microRNAs in individuals at risk of progression to rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 288.
- Yue, J.; Lau, T.C.K.; Griffith, J.F.; Xu, J.; Xiao, F.; Shi, L.; Wang, D.; Wong, P.C.H.; Li, E.K.; Tam, L.P.; et al. Circulating miR-99b-5p as a novel predictor of erosion progression on high-resolution peripheral quantitative computed tomography in early rheumatoid arthritis: A prospective cohort study. Int. J. Rheum. Dis. 2019, 22, 1724–1733.
- Cunningham, C.C.; Wade, S.; Floudas, A.; Orr, C.; McGarry, T.; Wade, S.; Cregan, S.; Fearon, U.; Veale, D.J. Serum miRNA Signature in Rheumatoid Arthritis and “At-Risk Individuals”. Front. Immunol. 2021, 12, 633201.
- Wu, Y.; Ai, H.; Zou, Y.; Xu, J. Osteoclast-derived extracellular miR-106a-5p promotes osteogenic differentiation and facilitates bone defect healing. Cell. Signal. 2023, 102, 110549.
- Zhang, J.; Liu, G.; Meng, Y.; Lin, H.; Lu, Y. MAG-2 promotes invasion, mobility and adherence capability of lung cancer cells by MMP-2, CD44 and intracellular calcium in vitro. Oncol. Rep. 2009, 21, 697–706.
- Anh, D.J.; Dimai, H.P.; Hall, S.L.; Farley, J.R. Skeletal alkaline phosphatase activity is primarily released from human osteoblasts in an insoluble form, and the net release is inhibited by calcium and skeletal growth factors. Calcif. Tissue Int. 1998, 62, 332–340.
- van Straalen, J.P.; Sanders, E.; Prummel, M.F.; Sanders, G.T.B. Bone-alkaline phosphatase as indicator of bone formation. Clin. Chim. Acta 1991, 201, 27–34.
- Huyan, T.; Du, Y.; Peng, H.; Hu, X.; Wu, C.; Li, Q.; Dong, D.; Li, J.; Shang, P. Evaluation of osteoclast-derived exosomal miRNA under simulated microgravity conditions using next-generation sequencing. Acta Astronaut. 2019, 161, 75–86.
- Chen, J.; Liu, M.; Luo, X.; Peng, L.; Zhao, Z.; He, C.; He, Y. Exosomal miRNA-486-5p derived from rheumatoid arthritis fibroblast-like synoviocytes induces osteoblast differentiation through the Tob1/BMP/Smad pathway. Biomater. Sci. 2020, 8, 3430–3442.
- Liu, H.; Dong, Y.; Feng, X.; Li, L.; Jiao, Y.; Bai, S.; Feng, Z.; Yu, H.; Li, X.; Zhao, Y. MiR-34a promotes bone regeneration in irradiated bone defects by enhancing osteoblastic differentiation of mesenchymal stromal cells in rats. Stem Cell Res. Ther. 2019, 10, 180.
- Ma, S.; Wang, D.D.; Ma, C.Y.; Zhang, Y.D. microRNA-96 promotes osteoblast differentiation and bone formation in ankylosing spondylitis mice through activating the Wnt signaling pathway by binding to SOST. J. Cell. Biochem. 2019, 120, 15429–15442.
- Qi, X.B.; Jia, B.; Wang, W.; Xu, G.H.; Guo, J.C.; Li, X.; Liu, J.N. Role of miR-199a-5p in osteoblast differentiation by targeting TET2. Gene 2020, 726, 144193.
- Wang, Y.X.; Peng, Z.L.; Sun, Z.W.; Pan, Y.J.; Ai, H.; Mai, Z.H. MiR-20a promotes osteogenic differentiation in bone marrow-derived mesenchymal stem/stromal cells and bone repair of the maxillary sinus defect model in rabbits. Front. Bioeng. Biotechnol. 2023, 11, 1127908.
- Yuan, H.; Li, M.; Feng, X.; Zhu, E.; Wang, B. miR-142a-5p promoted osteoblast differentiation via targeting nuclear factor IA. J. Cell. Physiol. 2021, 236, 1810–1821.
- Liang, M.; Yin, X.; Zhang, S.; Ai, H.; Luo, F.; Xu, J.; Dou, C.; Dong, S.; Ma, Q. Osteoclast-derived small extracellular vesicles induce osteogenic differentiation via inhibiting ARHGAP1. Mol. Ther. Nucleic Acids 2021, 23, 1191–1203.
- Yang, J.X.; Xie, P.; Li, Y.S.; Wen, T.; Yang, X.C. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell. Signal. 2020, 70, 109504.
- Gao, M.; Chen, M.; Li, C.; Xu, M.; Liu, Y.; Cong, M.; Sang, N.; Liu, S. Long non-coding RNA MT1DP shunts the cellular defense to cytotoxicity through crosstalk with MT1H and RhoC in cadmium stress. Cell Discov. 2018, 4, 5.
- Holliday, L.S.; McHugh, K.P.; Zuo, J.; Aguirre, J.I.; Neubert, J.K.; Rody, W.J., Jr. Exosomes: Novel regulators of bone remodelling and potential therapeutic agents for orthodontics. Orthod. Craniofacial Res. 2017, 20, 95–99.
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015.
- Li, D.; Liu, J.; Guo, B.; Liang, C.; Dang, L.; Lu, C.; He, X.; Cheung, H.Y.S.; Xu, L.; Lu, C.; et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat. Commun. 2016, 7, 10872.
- Zeng, Q.; Wang, Y.; Gao, J.; Yan, Z.; Li, Z.; Zou, X.; Li, Y.; Wang, J.; Guo, Y. miR-29b-3p regulated osteoblast differentiation via regulating IGF-1 secretion of mechanically stimulated osteocytes. Cell. Mol. Biol. Lett. 2019, 24, 11.
- Huynh, N.; Vonmoss, L.; Smith, D.; Rahman, I.; Felemban, M.F.; Zuo, J.; Rody, W.J.; McHugh, K.P.; Holliday, L.S. Characterization of regulatory extracellular vesicles from osteoclasts. J. Dent. Res. 2016, 95, 673–679.
- Stephens, E.; Roy, M.; Bisson, M.; Nguyen, H.D.; Scott, M.S.; Boire, G.; Bouchard, L.; Roux, S. Osteoclast signaling-targeting miR-146a-3p and miR-155-5p are downregulated in Paget’s disease of bone. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165852.
- Jia, X.; Yang, M.; Hu, W.; Cai, S. Overexpression of miRNA-22-3p attenuates osteoporosis by targeting MAPK14. Exp. Ther. Med. 2021, 22, 692.
- Kim, K.; Kim, J.H.; Kim, I.; Lee, J.; Seong, S.; Park, Y.; Kim, N. MicroRNA-26a Regulates RANKL-Induced Osteoclast Formation. Mol. Cells 2015, 38, 75–80.
- Lian, W.S.; Ko, J.Y.; Chen, Y.S.; Ke, H.J.; Hsieh, C.K.; Kuo, C.W.; Wang, S.Y.; Huang, B.W.; Tseng, J.G.; Wang, F.S. MicroRNA-29a represses osteoclast formation and protects against osteoporosis by regulating PCAF-mediated RANKL and CXCL12. Cell Death Dis. 2019, 10, 705.
- Minamizaki, T.; Nakao, Y.; Irie, Y.; Ahmed, F.; Itoh, S.; Sarmin, N.; Yoshioka, H.; Nobukiyo, A.; Fujimoto, C.; Niida, S.; et al. The matrix vesicle cargo miR-125b accumulates in the bone matrix, inhibiting bone resorption in mice. Commun. Biol. 2020, 3, 30.
- Wang, S.; Maruyama, E.O.; Martinez, J.; Lopes, J.; Hsu, T.; Wu, W.; Hsu, W.; Maruyama, T. miRNA-27a is essential for bone remodeling by modulating p62-mediated osteoclast signaling. Elife 2023, 12, e79768.




