
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Edward Araujo Júnior | -- | 2168 | 2024-02-07 21:08:49 | | | |
| 2 | Jason Zhu | Meta information modification | 2168 | 2024-02-08 02:48:39 | | |
Video Upload Options
Parvovirus B19, a member of the Parvoviridae family, is a human pathogenic virus. It can be transmitted by respiratory secretions, hand-to-mouth contact, blood transfusion, or transplacental transmission. Most patients are asymptomatic or present with mild symptoms such as erythema infectiosum, especially in children. In rare cases, moderate-to-severe symptoms may occur, affecting blood cells and other systems, resulting in anemia, thrombocytopenia, and neutropenia. Non-immune pregnant women are at risk for fetal infection by parvovirus B19, with greater complications if transmission occurs in the first or second trimester. Infected fetuses may not show any abnormalities in most cases, but in more severe cases, there may be severe fetal anemia, hydrops, and even pregnancy loss.
1. Maternal Infection—Clinical Presentation
2. Intrauterine Infection
2.1. Transmission
2.2. Pathophysiology
2.3. Effects of Infection on Pregnancy
- (1)
-
Spontaneous abortion: The rate of spontaneous abortion decreases with gestational age at diagnosis, with a rate of 13% before 20 weeks’ gestation and 0.5% after 20 weeks’ gestation [11][13]. The reason for this difference remains unclear, but the largest study suggests that it may be related to multiple organ damage, which can occur in the absence of anemia or hydrops, the most classic findings associated with fetal infection [18].
- (2)
-
Non-immune hydrops: The most obvious manifestation of congenital parvovirus B19 infection is fetal hydrops. The risk of hydrops is directly related to the gestational age when maternal infection occurs. If the infection occurs in the first trimester, the risk of hydrops varies from less than 5% to about 10%. If the infection occurs between 13 and 20 weeks, the risk of hydrops drops to 5% or less. If the infection occurs after 20 weeks’ gestation, the risk of fetal hydrops is 1% or less [7][19][20].
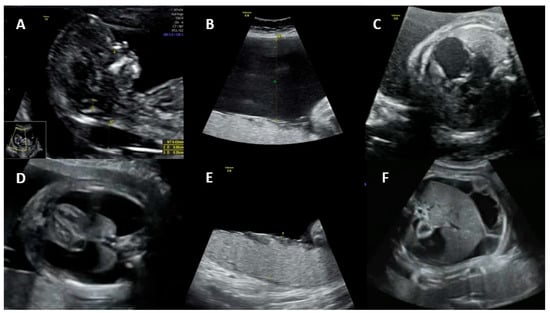
3. Maternal Diagnosis
4. Fetal Diagnosis
4.1. Diagnosis of Infection
4.2. Diagnosis of Fetal Anemia
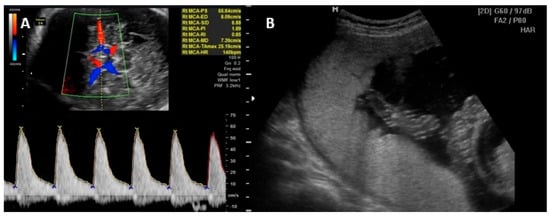
5. Prognosis
References
- Garcia, R.D.C.N.C.; Pereira, R.F.A.; Azevedo, K.M.L.D.; Castro, T.X.D.; Mello, F.C.; Setubal, S.; Oliveira, S.A.D. Molecular diversity of human parvovirus B19 during two outbreaks of erythema infectiosum in Brazil. Braz. J. Infect. Dis. 2017, 21, 102–106.
- Ishikawa, A.; Yoto, Y.; Tsugawa, T.; Tsutsumi, H. Quantitation of human parvovirus B19 DNA in erythema infectiosum and aplastic crisis. J. Med. Virol. 2014, 86, 2102–2106.
- Gigi, C.E.; Anumba, D.O.C. Parvovirus b19 infection in pregnancy—A review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 264, 358–362.
- Chisaka, H.; Ito, K.; Niikura, H.; Sugawara, J.I.; Takano, T.; Murakami, T.; Yaegashi, N. Clinical manifestations and outcomes of parvovirus B19 infection during pregnancy in Japan. Tohoku J. Exp. Med. 2006, 209, 277–283.
- Dukes, C. On the confusion of two different diseases under the name rubella (rose-rash). Lancet 1900, 156, 89–95.
- Khan, U.; Uzair Ahmad, R.; Ullah, Z.; Fida, T.; Shehryar, M. Parvovirus b19-Induced Acute Hepatitis with Hepatosplenomegaly and Polyarthropathy. Cureus 2022, 14, e21494.
- Harger, J.H.; Adler, S.P.; Koch, W.C.; Harger, G.F. Prospective evaluation of 618 pregnant women exposed to parvovirus B19: Risks and symptoms. Obstet. Gynecol. 1998, 91, 413–420.
- Lamont, R.F.; Sobel, J.D.; Vaisbuch, E.; Kusanovic, J.P.; Mazaki-Tovi, S.; Kim, S.K.; Romero, R. Parvovirus B19 infection in human pregnancy. BJOG 2011, 118, 175–186.
- Obeid Mohamed, S.O.; Osman Mohamed, E.M.; Ahmed Osman, A.A.; Mohamed, A.; Elmugadam, F.A.; Abdalla Ibrahim, G.A. A Meta-Analysis on the Seroprevalence of Parvovirus B19 among Patients with Sickle Cell Disease. Biomed Res. Int. 2019, 2019, 2757450.
- Oiwa, H.; Shimada, T.; Hashimoto, M.; Kawaguchi, A.; Ueda, T.; Sugiyama, E.; Kamiya, T. Clinical findings in parvovirus B19 infection in 30 adult patients in Kyoto. Mod. Rheumatol. 2011, 21, 24–31.
- Public Health Laboratory Service Working Party on Fifth Disease. Prospective study of human parvovirus (B19) infection in pregnancy. BMJ 1990, 300, 1166–1170.
- Levy, R.; Weissman, A.; Blomberg, G.; Hagay, Z.J. Infection by parvovirus B19 during pregnancy: A review. Obstet. Gynecol. Surv. 1997, 52, 254–259.
- Miller, E.; Fairley, C.K.; Cohen, B.J.; Seng, C. Immediate and longterm outcome of human parvovirus B19 infection in pregnancy. Br. J. Obstet. Gynaecol. 1998, 105, 174–178.
- Schild, R.L.; Bald, R.; Plath, H.; Eis-Hübinger, A.M.; Enders, G.; Hansmann, M. Intrauterine management of fetal parvovirus B19 infection. Ultrasound Obs. Gynecol. 1999, 13, 161–166.
- Broliden, K.; Tolfvenstam, T.; Norbeck, O. Clinical aspects of parvovirus B19 infection. J. Intern. Med. 2006, 260, 285–304.
- Hoffman, R.; Benz, E.J., Jr.; Silberstein, L.E.; Heslop, H.E.; Weitz, J.I.; Anastasi, J.; Salama, M.E.; Abutalib, S. Hematology: Basic Principles and Practice, 5th ed.; Churchill Livingstone, An Imprint of Elsevier: Philadelphia, PA, USA, 2009.
- Bascietto, F.; Liberati, M.; Murgano, D.; Buca, D.; Iacovelli, A.; Flacco, M.E.; D’Antonio, F. Outcome of fetuses with congenital parvovirus B19 infection: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 52, 569–576.
- de Jong, E.P.; Walther, F.J.; Kroes, A.C.; Oepkes, D. Parvovirus B19 infection in pregnancy: New insights and management. Prenat. Diagn. 2011, 31, 419–425.
- Puccetti, C.; Contoli, M.; Bonvicini, F.; Cervi, F.; Simonazzi, G.; Gallinella, G.; Rizzo, N. Parvovirus B19 in pregnancy: Possible consequences of vertical transmission. Prenat. Diagn. 2012, 32, 897–902.
- Hayakawa, H.; Tara, M.; Niina, K.; Osame, M. A clinical study of adult human parvovirus B19 infection. Intern. Med. 2002, 41, 295–299.
- Fairley, C.K.; Smoleniec, J.S.; Caul, O.E.; Miller, E. Observational study of effect of intrauterine transfusions on outcome of fetal hydrops after parvovirus B19 infection. Lancet 1995, 346, 1335–1337.
- Katz, V.; McCoy, M.; Kuller, J.; Hansen, W. An association between fetal parvovirus B19 infection and fetal anomalies: A report of two cases. Am. J. Perinatol. 1996, 13, 43–45.
- Attwood, L.O.; Holmes, N.E.; Hui, L. Identification and management of congenital parvovirus B19 infection. Prenat. Diagn. 2020, 40, 1722–1731.
- Schwarz, T.F.; Jäger, G.; Gilch, S. Comparison of seven commercial tests for the detection of parvovirus B19-specific IgM. Zentralbl. Bakteriol. 1997, 285, 525–530.
- Voordouw, B.; Rockx, B.; Jaenisch, T.; Fraaij, P.; Mayaud, P.; Vossen, A.; Koopmans, M. Performance of Zika Assays in the Context of Toxoplasma gondii, Parvovirus B19, Rubella Virus, and Cytomegalovirus (TORCH) Diagnostic Assays. Clin. Microbiol. Rev. 2019, 33, e00130-18.
- Crane, J.; Mundle, W.; Boucoiran, I.; Maternal Fetal Medicine Committee. Parvovirus B19 infection in pregnancy. J. Obstet. Gynaecol. Can. 2014, 36, 1107–1116.
- Dijkmans, A.C.; de Jong, E.P.; Dijkmans, B.A.; Lopriore, E.; Vossen, A.; Walther, F.J.; Oepkes, D. Parvovirus B19 in pregnancy: Prenatal diagnosis and management of fetal complications. Curr. Opin. Obstet. Gynecol. 2012, 24, 95–101.
- Giorgio, E.; De Oronzo, M.A.; Iozza, I.; Di Natale, A.; Cianci, S.; Garofalo, G.; Giacobbe, A.M.; Politi, S. Parvovirus B19 during pregnancy: A review. J. Prenat. Med. 2010, 4, 63–66.
- Dieck, D.; Schild, R.L.; Hansmann, M.; Eis-Hübinger, A.M. Prenatal diagnosis of congenital parvovirus B19 infection: Value of serological and PCR techniques in maternal and fetal serum. Prenat. Diagn. 1999, 19, 1119–1123.
- Mari, G.; Deter, R.L.; Carpenter, R.L.; Rahman, F.; Zimmerman, R.; Moise, K.J., Jr.; Blackwell, S.C. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia due to maternal red-cell alloimmunization. Collaborative Group for Doppler Assessment of the Blood Velocity in Anemic Fetuses. New Engl. J. Med. 2000, 342, 9–14.
- Borna, S.; Mirzaie, F.; Hanthoush-Zadeh, S.; Khazardoost, S.; Rahimi-Sharbaf, F. Middle cerebral artery peak systolic velocity and ductus venosus velocity in the investigation of nonimmune hydrops. J. Clin. Ultrasound 2009, 37, 385–388.
- Cosmi, E.; Mari, G.; Delle Chiaie, L.; Detti, L.; Akiyama, M.; Murphy, J.; Bahado-Singh, R. Noninvasive diagnosis by Doppler ultrasonography of fetal anemia resulting from parvovirus infection. Am. J. Obstet. Gynecol. 2002, 187, 1290–1293.
- Delle Chiaie, L.; Buck, G.; Grab, D.; Terinde, R. Prediction of fetal anemia with Doppler measurement of the middle cerebral artery peak systolic velocity in pregnancies complicated by maternal blood group alloimmunization or parvovirus B19 infection. Ultrasound Obstet. Gynecol. 2001, 18, 232–236.
- Yaron, Y.; Hassan, S.; Geva, E.; Kupferminc, M.J.; Yavetz, H.; Evans, M.I. Evaluation of fetal echogenic bowel in the second trimester. Fetal Diagn. Ther. 1999, 14, 176–180.
- Travan, L.; Naviglio, S.; Cont, G.; Brovedani, P.; Davanzo, R.; Demarini, S. Isolated hypoplasia of abdominal wall muscles associated with fetal ascites. Congenit. Anom. 2016, 56, 184–186.
- Grubman, O.; Hussain, F.N.; Nelson, Z.; Brustman, L. Maternal Parvovirus B19 Infection Causing First-Trimester Increased Nuchal Translucency and Fetal Hydrops. Case Rep. Obstet. Gynecol. 2019, 2019, 3259760.
- Lassen, J.; Jensen, A.K.; Bager, P.; Pedersen, C.B.; Panum, I.; Nørgaard-Pedersen, B.; Melbye, M. Parvovirus B19 infection in the first trimester of pregnancy and risk of fetal loss: A population-based case-control study. Am. J. Epidemiol. 2012, 176, 803–807.
- Xiong, Y.Q.; Tan, J.; Liu, Y.M.; He, Q.; Li, L.; Zou, K.; Sun, X. The risk of maternal parvovirus B19 infection during pregnancy on fetal loss and fetal hydrops: A systematic review and meta-analysis. J. Clin. Virol. 2019, 114, 12–20.
- Enders, M.; Weidner, A.; Zoellner, I.; Searle, K.; Enders, G. Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: Prospective evaluation of 1018 cases. Prenat. Diagn. 2004, 24, 513–518.
- Pistorius, L.R.; Smal, J.; De Haan, T.R.; Page-Christiaens, G.C.; Verboon-Maciolek, M.; Oepkes, D.; De Vries, L.S. Disturbance of cerebral neuronal migration following congenital parvovirus B19 infection. Fetal Diagn. Ther. 2008, 24, 491–494.
- Schulert, G.S.; Walsh, W.F.; Weitkamp, J.H. Polymicrogyria and congenital parvovirus b19 infection. AJP Rep. 2011, 1, 105–110.
- Lindenburg, I.T.; van Klink, J.M.; Smits-Wintjens, V.E.; van Kamp, I.L.; Oepkes, D.; Lopriore, E. Long-term neurodevelopmental and cardiovascular outcome after intrauterine transfusions for fetal anaemia: A review. Prenat. Diagn. 2013, 33, 815–822.





