
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Anil Kumar Madikere Raghunatha Reddy | -- | 6341 | 2024-02-07 19:31:37 | | | |
| 2 | Sirius Huang | Meta information modification | 6341 | 2024-02-08 03:00:51 | | |
Video Upload Options
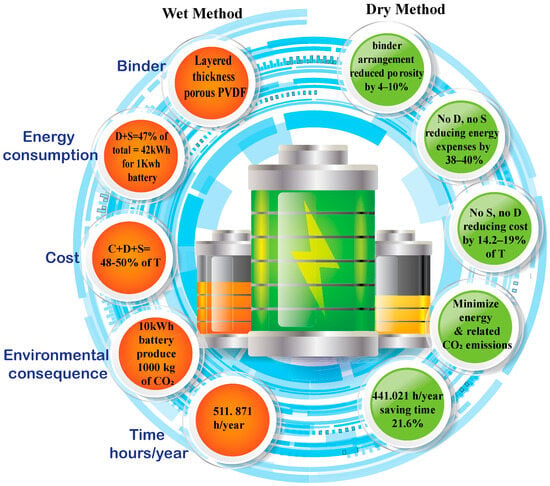
The pursuit of industrializing lithium-ion batteries (LIBs) with exceptional energy density and top-tier safety features presents a substantial growth opportunity. The demand for energy storage is steadily rising, driven primarily by the growth in electric vehicles and the need for stationary energy storage systems. However, the manufacturing process of LIBs, which is crucial for these applications, still faces significant challenges in terms of both financial and environmental impacts. The dry process procedure aims to reduce the risk of solvent emissions, waste generation, and potential safety hazards by eliminating the need for solvents. This aligns with a growing emphasis on sustainable and environmentally friendly manufacturing practices.
1. Introduction
2. Dry Electrode Processing for LIBs
2.1. Electrodes
| Ref. | Process | Electrode | Materials Wt. % |
Thickness (µm) | Porosity (%) |
Area Capacity (AC) (mAh/cm2), Mass Loading (ML) (mg/cm2) | Discharge Capacity (mAh/g) | Capacity Retention (%) |
|---|---|---|---|---|---|---|---|---|
| [22] | Wet | Cathode | NMC111:PVDF:CB 90:5:5 |
- | 30 | - | 138 | 84%, after undergoing 50 rounds of charging and discharging at a 0.5 C rate within the voltage range of 2.8–4.3 V |
| Dry | NMC111:PVDF:CB 90:5:5 |
40–130 | 30 | - | 138 | 87%, after 50 cycles at 0.5 C between 2.8 and 4.3 V | ||
| Wet | LCO:PVDF:CB 90:5:5 |
- | 30 | - | 115 | 58%, after undergoing 50 rounds of charging and discharging at a 0.5 C rate within the voltage range of 2.5–4.2 V | ||
| Dry | LCO:PVDF:CB 90:5:5 |
40–130 | 30 | - | 114 | 70%, after 50 cycles at 0.5 C between 2.5 and 4.2 V | ||
| [42] | Wet | NMC111:PVD:CB 19:1:1 |
32.6 | 41 | 7.65 mg/cm2 (ML) | 156 | 60%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | |
| 52 | 35 | 14.27 mg/cm2 (ML) | 157 | 65%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | ||||
| Dry | NMC111:PVDF:CB 19:1:1 wt. |
40.5 | 31 | 10.07 mg/cm2 (ML) | 155 | 80%, after 300 cycles between 3.0 V and 4.3 V at 0.5 C | ||
| [23] | Wet | NMC11:PVDF:CB 90:5:5 |
55 | 29–30 | 2.45m Ah/cm2 (AC) | 145 | 65%, after 500 cycles at 0.5 C. | |
| Dry | NMC111:PVDF:CB90:5:5 | 55 100 150 200 |
29–30 | 2.45 mAh/cm2 (AC) 5.80 mAh/cm2 (AC) 6.52 mAh/cm2 (AC) 9.11 mAh/cm2 (AC) |
150 <20 <20 <20 At 3 C. |
80%, after 500 cycles at 0.5 C. | ||
| [29] | Wet | Anode | Graphite:PVDF:CB 85:10:5 |
- | - | PVDF 3 mAh/cm2 (AC) | - | - |
| Graphite:(FEP or TVH):CB 86:7:7 | ||||||||
| Dry | Graphite:PVDF:CB 85:10:5 |
- | - | FEP 2.7 mAh/cm2 (AC) | 370 | 99%, after 50 cycles at 0.5 C | ||
| Graphite:(FEP or TVH):CB 86:7:7 | TVH 3.5m Ah/cm2 (AC) | 345 | 97%, after 50 cycles at 0.5 C |
2.2. Dry Mixing and Coating
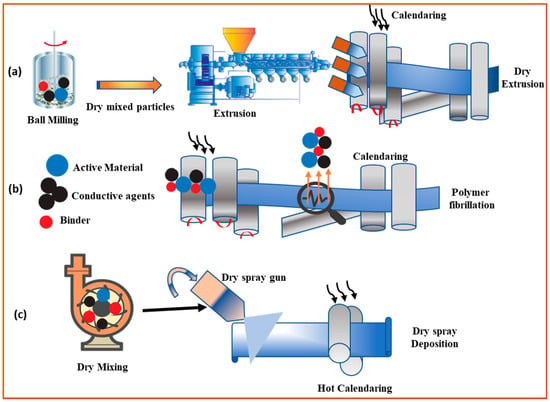
2.3. Dry Spraying Deposition
| Parameter | Dry-Painted Electrodes | Conventional Wet Electrodes | Implications |
|---|---|---|---|
| Cyclic voltammetry (CV) at 0.025 mV/s | Single pair of oxidation and reduction peaks (reduction at ~3.8 V, oxidation at ~4 V) | Single pair of oxidation and reduction peaks (reduction at ~3.8 V, oxidation at ~4 V) | Both types of electrodes show good reversibility of lithium insertion and extraction from LCO |
| CV peak symmetry at higher scan rates | Maintain symmetrical shape of cathodic and anodic peaks | Significant change in the shape of cathodic and anodic peaks | Dry-painted electrodes exhibit better rate capabilities and stability under rapid charge/discharge conditions |
| Potential difference between peaks | Smaller potential difference between cathodic and anodic peaks | The larger potential difference between cathodic and anodic peaks | Dry-painted electrodes have lower electrochemical polarization, indicating more efficient electrochemical reactions |
| EIS analysis | Show lower impedance characteristics | Show higher impedance characteristics | Lower impedance in dry-painted electrodes suggests better conductivity and lower internal resistance |
| Charge transfer resistance | Lower | Higher | Lower charge transfer resistance in dry-painted electrodes implies more efficient electron transfer during electrochemical reactions |
| Lithium-ion diffusion | Potentially more favorable | Potentially less favorable | Better lithium-ion diffusion in painted electrodes could contribute to their enhanced performance |
-
Wet (lower loading): the electrode had a thickness of 32.6 µm, and it carried a mass loading value of 7.65 mg/cm2;
-
Wet (higher loading): the electrode had a thickness of 52 µm, with a mass loading of 14.27 mg/cm2;
-
Dry: the dry electrode had a thickness of 40.5 µm, with a mass loading of 10.07 mg/cm2.
-
Wet (lower loading): the electrodes exhibited an initial discharge capacity of 156 mAh/g, with a capacity retention of 60% after 300 cycles when cycled between 3 V and 4.3 V at a rate of 0.5 C;
-
Wet (higher loading): the electrode demonstrated an initial discharge capacity of 157 mAh/g, with a capacity retention of 65% after the same cycling conditions;
-
Dry: the dry electrode displayed a primary discharge capability retention of 80% after the same cycling conditions.
-
Wet (55 µm): the electrode showed a primary discharge of 145 mAh/g, with a capacity retention of 65% after 500 cycles;
-
Dry (55 µm): the electrode exhibited an initial discharge capacity of 150 mAh/g, with capacity retention of over 80% after the same cycling conditions;
-
Dry (55 µm): the electrode displayed a discharge capacity of 120 mAh/g at a high rate of 3 C;
-
Dry (100 µm, 150 µm, and 200 µm): the electrode exhibited a discharge capacity of less than 20 mAh/g at the same high rate of 3 C.
-
Low MW PVDF: the electrode displayed an initial discharge capacity of 160 mAh/g at a rate of 0.2 C, with capacity retention of 93% after 50 cycles at 0.5 C; however, at a higher rate of 5 C, the capacity retention dropped to 16.7%;
-
High MW PVDF: the electrode exhibits a primary discharge capacity of 160 mAh/g at 0.2 C, with a capacity retention of 91% following 50 cycles.
2.4. Polymer Fibrillation
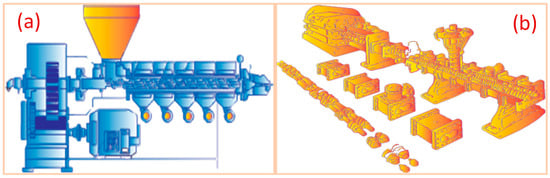
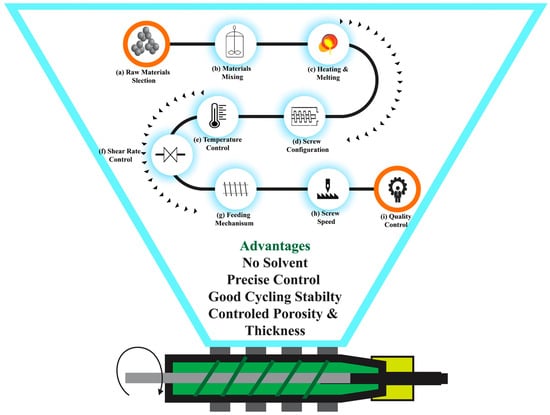
2.5. Extrusion and Melt Processing
| Criteria | With Solvent [95][96] | Without Solvent [61][88][94] |
|---|---|---|
| Manufacturing process | Involves slurry-casting procedure | Utilizes melt extrusion technique |
| Active materials | Limited option | Diverse (LFP, NMC, LTO, NCA, …) |
| Binders | Conventional binders | PPC, HNRP,… |
| Thickness | Varies | Up to 500 µm |
| Density | - | ~2.9 g/cm3 |
| Volumetric capacity | - | ~349 mAh/cm3 |
| Areal capacity | - | >15.2 mAh/cm2 |
| Porosity | Uncontrolled | Controlled |
| Electrochemical performance | Varies | Enhanced |
| Dry Method | Process Description | Active Materials | Binder and Proprieties | Temperature | Chief Performance | References | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|---|
| Dry spray deposition | Utilizes a spraying gun | NMC, LCO | PVDF Thermoplastic |
100–190 °C 250 °C |
Increased bonding strength | [22][24] |
|
|
| NMC | PVDF Thermoplastic |
170 °C | 80%: highest capacity retention after 300 cycles | [42] | ||||
| NMC | PVDF Thermoplastic |
180 °C | (>650 cycles) at a standard thickness of 56 µm. Electrodes (up to 200 µm) |
[23] | ||||
| NMC | PVDF Thermoplastic |
200 °C | High molecular weight: PVDF is preferred | [59] | ||||
| Graphite | FEP/THV | 170–300 °C | New binders. High electrochemical performance | [29] | ||||
| Polymer fibrillation | Utilization of fibrillizable PTFE | LFP, LTO + 40% AC. | PTFE | 160–180 °C | Higher capacity and energy density | [98] |
|
|
| NMC, graphite Silicon/graphite |
PTFE | 120 °C | High loading: 5 mAh/cm2, thick electrode, high-rate capability | [28] | ||||
| Graphite, hard carbon, soft carbon | PTFE | 160–180 °C | Excellent cycle life and high stability of hard and soft carbon | [81] | ||||
| NMC, CNF | PTFE | 320–330 °C | 100 charge–discharge cycles without artificial pressure, high areal 6.5 mAh/cm2. | [31] | ||||
| Extrusion and melt process |
Extruded to form a continuous, uniform electrode film | LFP | PP PW SA Thermoplastic |
160–190 °C | Cathode exhibits good cyclability across 20 cycles at a C/10 rate—high areal capacity (13.7 mAh/cm−2) with 500 µm thickness. | [91] |
|
|
| LFP, NMC LTO |
PPC elastomeric HNBR | 40–90 °C | A new binder, such as HNBR, decreases the viscosity, control of thickness, and porosity. | [94] | ||||
| NCA, graphite | PPC elastomeric | 50–250 °C | Areal capacities over 5 mAh/cm2 at a C/5 rate, large loading range from 4–40 mg/cm2. | [99] |
References
- Toro, L.; Moscardini, E.; Baldassari, L.; Forte, F.; Falcone, I.; Coletta, J.; Toro, L. A Systematic Review of Battery Recycling Technologies: Advances, Challenges, and Future Prospects. Energies 2023, 16, 6571.
- Zheng, M.; Salim, H.; Liu, T.; Stewart, R.A.; Lu, J.; Zhang, S. Intelligence-Assisted Predesign for the Sustainable Recycling of Lithium-Ion Batteries and Beyond. Energy Environ. Sci. 2021, 14, 5801–5815.
- Shu, X.; Guo, Y.; Yang, W.; Wei, K.; Zhu, G. Life-cycle assessment of the environmental impact of the batteries used in pure electric passenger cars. Energy Rep. 2021, 7, 2302–2315.
- Nagaura, T. Lithium ion rechargeable battery. Prog. Batteries Sol. Cells 1990, 9, 209.
- Broadhead, J.; Gibbard, H.F.; Kuo, H.; Chi, I.; Bowden, W. High-Energy, High-Power Lithium-Ion Rechargeable Cells. In Proceedings of the Symposium on Rechargeable Lithium and Lithium-Ion Batteries; The Electrochemical Society: Pennington, NJ, USA, 1995; Volume 94, p. 370.
- Ram, M.; Aghahosseini, A.; Breyer, C. Job creation during the global energy transition towards 100% renewable power system by 2050. Technol. Forecast. Soc. Change 2020, 151, 119682.
- Degen, F.; Krätzig, O. Future in Battery Production: An Extensive Benchmarking of Novel Production Technologies as Guidance for Decision Making in Engineering. IEEE Trans. Eng. Manag. 2022, 71, 1038–1056.
- Global EV Outlook 2023. Available online: https://iea.blob.core.windows.net/assets/dacf14d2-eabc-498a-8263-9f97fd5dc327/GEVO2023.pdf (accessed on 5 May 2023).
- World Economic Forum. A Vision for a Sustainable Battery Value Chain in 2030. Available online: https://www3.weforum.org/docs/WEF_A_Vision_for_a_Sustainable_Battery_Value_Chain_in_2030_Report.pdf (accessed on 9 May 2023).
- Degen, F. Lithium-Ion Battery Cell Production in Europe: Scenarios for Reducing Energy Consumption and Greenhouse Gas Emissions Until 2030. J. Ind. Ecol. 2023, 27, 964–976.
- Musk, E. Tesla Battery Day 2020; Tesla, Inc.: Fremont, CA, USA, 2020; Available online: https://www.tesla.com/2020shareholdermeeting (accessed on 10 May 2023).
- Wang, M.; Dong, X.; Escobar, I.C.; Cheng, Y.T. Lithium Ion Battery Electrodes Made Using Dimethyl Sulfoxide (DMSO) A Green Solvent. ACS Sustain. Chem. Eng. 2020, 8, 11046–11051.
- Haufroid, V.; Jaeger, V.K.; Jeggli, S.; Eisenegger, R.; Bernard, A.; Friedli, D.; Lison, D.; Hotz, P. Biological Monitoring and Health Effects of Low-Level Exposure to N-Methyl-2-Pyrrolidone: A Cross-Sectional Study. Int. Arch. Occup. Environ. Health 2014, 87, 663–674.
- Zhang, R.; Shi, X.; Esan, O.C.; An, L. Organic Electrolytes Recycling From Spent Lithium-Ion Batteries. Glob. Chall. 2022, 6, 2200050.
- Ryu, M.; Hong, Y.K.; Lee, S.Y.; Park, J.H. Ultrahigh Loading Dry-Process for Solvent-Free Lithium-Ion Battery Electrode Fabrication. Nat. Commun. 2023, 14, 1316.
- Chordia, M.; Nordelöf, A.; Ellingsen, L.A.W. Environmental Life Cycle Implications of Upscaling Lithium-Ion Battery Production. Int. J. Life Cycle Assess. 2021, 26, 2024–2039.
- Fernandez-Diaz, L.; Castillo, J.; Sasieta-Barrutia, E.; Arnaiz, M.; Cabello, M.; Judez, X.; Villaverde, A. Mixing Methods for Solid State Electrodes: Techniques, Fundamentals, Recent Advances, and Perspectives. Chem. Eng. J. 2023, 464, 142469.
- Valikangas, J.; Sliz, R.; Silva Santos, H.; Vilmi, P.; Rieppo, L.; Hu, T.; Fabritius, T. Suitable Cathode NMP Replacement for Efficient Sustainable Printed Li-Ion Batteries. ACS Appl. Energy Mater. 2022, 5, 4047–4058.
- Daniel, C.; Li, J.; Mohanty, D.; Wood, D.L., III. Thick Low-Cost, High-Power Lithium-Ion Electrodes via Aqueous Processing. In Proceedings of the US Department of Energy Hydrogen and Fuel Cells Program Annual Merit Review and Peer Evaluation Meeting, Washington, DC, USA, 6–10 June 2016; Office of Energy Efficiency & Renewable Energy: Washington, DC, USA, 2016.
- Lyckfeldt, O.; Orlenius, J.; Kasvayee, K.A.; Johander, P. Water Based Processing of LiFePO4/C Cathode Material for Li-Ion Batteries Utilizing Freeze Granulation. J. Power Sources 2012, 213, 119–127.
- Chen, C.F.; Stein, M., IV; Robles, D.J.; Rhodes, C.; Mukherjee, P.P. Non-Aqueous Electrode Processing and Construction of Lithium-Ion Coin Cells. JoVE (J. Vis. Exp.) 2016, 108, e53490.
- Ludwig, B.; Zheng, Z.; Shou, W.; Wang, Y.; Pan, H. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries. Sci. Rep. 2016, 6, 23150.
- Liu, J.; Ludwig, B.; Liu, Y.; Zheng, Z.; Wang, F.; Tang, M.; Wang, Y. Scalable Dry Printing Manufacturing to Enable Long-Life and High Energy Lithium-Ion Batteries. Adv. Mater. Technol. 2017, 2, 1700106.
- Ludwig, B.; Liu, J.; Chen, I.M.; Liu, Y.; Shou, W.; Wang, Y.; Pan, H. Understanding Interfacial-Energy-Driven Dry Powder Mixing for Solvent-Free Additive Manufacturing of Li-Ion Battery Electrodes. Adv. Mater. Interfaces 2017, 4, 1700570.
- Wood, D.L., III; Wood, M.; Li, J.; Du, Z.; Ruther, R.E.; Hays, K.A.; Belharouak, I. Perspectives on the Relationship Between Materials Chemistry and Roll-to-Roll Electrode Manufacturing for High-Energy Lithium-Ion Batteries. Energy Storage Mater. 2020, 29, 254–265.
- Kato, Y.; Shiotani, S.; Morita, K.; Suzuki, K.; Hirayama, M.; Kanno, R. All-Solid-State Batteries with Thick Electrode Configurations. J. Phys. Chem. Lett. 2018, 9, 607–613.
- Shin, J.; Duong, H. Electrochemical Performance of Dry Battery Electrode. ECS Meet. Abstr. 2018, 233, 365.
- Duong, H.; Shin, J.; Yudi, Y. Dry Electrode Coating Technology. In Proceedings of the 48th Power Sources Conference, Denver, CO, USA, 11–14 June 2018; Volume 3, pp. 34–37.
- Schälicke, G.; Landwehr, I.; Dinter, A.; Pettinger, K.H.; Haselrieder, W.; Kwade, A. Solvent-Free Manufacturing of Electrodes for Lithium-Ion Batteries via Electrostatic Coating. Energy Technol. 2020, 8, 1900309.
- Shin, J.; Yudi, Y.; Magsino, P.; Wong, W.; Duong, H. Dry Processed Nickel-Rich Layered Transition Metal Oxide Cathode Electrode. ECS Meet. Abstr. 2019, 235, 317.
- Hippauf, F.; Schumm, B.; Doerfler, S.; Althues, H.; Fujiki, S.; Shiratsuchi, T.; Kaskel, S. Overcoming Binder Limitations of Sheet-Type Solid-State Cathodes Using a Solvent-Free Dry-Film Approach. Energy Storage Mater. 2019, 21, 390–398.
- Sahore, R.; Wood, D.L., III; Kukay, A.; Grady, K.M.; Li, J.; Belharouak, I. Towards Understanding of Cracking During Drying of Thick Aqueous-Processed LiNi0.8Mn0.1Co0.1O2 Cathodes. ACS Sustain. Chem. Eng. 2020, 8, 3162–3169.
- Lacey, S.D.; Walsh, E.D.; Hitz, E.; Dai, J.; Connell, J.W.; Hu, L.; Lin, Y. Highly Compressible, Binderless and Ultrathick Holey Graphene-Based Electrode Architectures. Nano Energy 2017, 31, 386–392.
- Hawley, W.B.; Parejiya, A.; Bai, Y.; Meyer, H.M., III; Wood, D.L., III; Li, J. Lithium and Transition Metal Dissolution Due to Aqueous Processing in Lithium-Ion Battery Cathode Active Materials. J. Power Sources 2020, 466, 228315.
- Wu, X.; Xia, S.; Huang, Y.; Hu, X.; Yuan, B.; Chen, S.; Liu, W. High-Performance, Low-Cost, and Dense-Structure Electrodes with High Mass Loading for Lithium-Ion Batteries. Adv. Funct. Mater. 2019, 29, 1903961.
- Hawley, W.B.; Li, J. Electrode manufacturing for lithium-ion batteries—Analysis of current and next generation processing. J. Energy Storage 2019, 25, 100862.
- Liu, Y.; Zhang, R.; Wang, J.; Wang, Y. Current and future lithium-ion battery manufacturing. iScience 2021, 24, 102332.
- Nelson, P.A.; Ahmed, S.; Gallagher, K.G.; Dees, D.W. Modeling the Performance and Cost of Lithium-Ion Batteries for Electric-Drive Vehicles; ANL/CSE-19/2; Argonne National Laboratory: Argonne, IL, USA, 2019.
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing cost of lithium-ion batteries. J. Power Sources 2015, 275, 234–242.
- Ahmed, S.; Nelson, P.A.; Gallagher, K.G.; Dees, D.W. Energy impact of cathode drying and solvent recovery during lithium-ion battery manufacturing. J. Power Sources 2016, 322, 169–178.
- Yuan, C.; Deng, Y.; Li, T.; Yang, F. Manufacturing energy analysis of lithium ion battery pack for electric vehicles. CIRP Ann. 2017, 66, 53–56.
- Al-Shroofy, M.; Zhang, Q.; Xu, J.; Chen, T.; Kaur, A.P.; Cheng, Y.T. Solvent-free dry powder coating process for low-cost manufacturing of LiNi1/3Mn1/3Co1/3O2 cathodes in lithium-ion batteries. J. Power Sources 2017, 352, 187–193.
- Wang, C.; Yu, R.; Duan, H.; Lu, Q.; Li, Q.; Adair, K.R.; Sun, X. Solvent-free approach for interweaving freestanding and ultrathin inorganic solid electrolyte membranes. ACS Energy Lett. 2021, 7, 410–416.
- Zackrisson, M.; Avellán, L.; Orlenius, J. Life cycle assessment of lithium-ion batteries for plug-in hybrid electric vehicles–Critical issues. J. Clean. Prod. 2010, 18, 1519–1529.
- Stein, M.; Mistry, A.; Mukherjee, P.P. Mechanistic understanding of the role of evaporation in electrode processing. J. Electrochem. Soc. 2017, 164, A1616.
- Radloff, S.; Carbonari, G.; Scurtu, R.G.; Hölzle, M.; Wohlfahrt-Mehrens, M. Advances in the Aqueous Processing of Ni-Rich Positive Electrodes. Electrochem. Soc. Meet. Abstr. 2022, 242, 284.
- Aranmala, K.; Chanhaew, A.; Rahmawati, M.; Ikhsanudin, M.N.; Meethong, N. Effects of Conductive Agents on Electrochemical Performance of Water-Based LiNi0.6Mn0.2Co0.2O2 Cathodes for Cylindrical Cell Production of Lithium-Ion Batteries. Defect Diffus. Forum 2022, 417, 169–176.
- Bi, J.; Du, Z.; Sun, J.; Liu, Y.; Wang, K.; Du, H.; Huang, W. On the Road to the Frontiers of Lithium-Ion Batteries: A Review and Outlook of Graphene Anodes. Adv. Mater. 2023, 35, 2210734.
- Kirsch, D.J.; Lacey, S.D.; Kuang, Y.; Pastel, G.; Xie, H.; Connell, J.W.; Hu, L. Scalable dry processing of binder-free lithium-ion battery electrodes enabled by holey graphene. ACS Appl. Energy Mater. 2019, 2, 2990–2997.
- Rollag, K.; Juarez-Robles, D.; Du, Z.; Wood, D.L., III; Mukherjee, P.P. Drying temperature and capillarity-driven crack formation in aqueous processing of Li-ion battery electrodes. ACS Appl. Energy Mater. 2019, 2, 4464–4476.
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29.
- Yan, Z.; Jiang, J.; Zhang, Y.; Yang, D.; Du, N. Scalable and low-cost synthesis of porous silicon nanoparticles as high-performance lithium-ion battery anode. Mater. Today Nano 2022, 18, 100175.
- Chouchane, M.; Rucci, A.; Lombardo, T.; Ngandjong, A.C.; Franco, A.A. Lithium ion battery electrodes predicted from manufacturing simulations: Assessing the impact of the carbon-binder spatial location on the electrochemical performance. J. Power Sources 2019, 444, 227285.
- Jaiser, S.; Funk, L.; Baunach, M.; Scharfer, P.; Schabel, W. Experimental investigation into battery electrode surfaces: The distribution of liquid at the surface and the emptying of pores during drying. J. Colloid Interface Sci. 2017, 494, 22–31.
- Erakca, M.; Baumann, M.; Bauer, W.; de Biasi, L.; Hofmann, J.; Bold, B.; Weil, M. Energy flow analysis of laboratory scale lithium-ion battery cell production. iScience 2021, 24, 102437.
- Kallitsis, E.; Korre, A.; Kelsall, G.; Kupfersberger, M.; Nie, Z. Environmental life cycle assessment of the production in China of lithium-ion batteries with nickel-cobalt-manganese cathodes utilising novel electrode chemistries. J. Clean. Prod. 2020, 254, 120067.
- Lu, Y.; Zhao, C.Z.; Yuan, H.; Hu, J.K.; Huang, J.Q.; Zhang, Q. Dry electrode technology, the rising star in solid-state battery industrialization. Matter 2022, 5, 876–898.
- Zanotto, F.M.; Dominguez, D.Z.; Ayerbe, E.; Boyano, I.; Burmeister, C.; Duquesnoy, M.; Franco, A.A. Data specifications for battery manufacturing digitalization: Current status, challenges, and opportunities. Batteries Supercaps 2022, 5, e202200224.
- Wang, M.; Hu, J.; Wang, Y.; Cheng, Y.T. The influence of polyvinylidene fluoride (PVDF) binder properties on LiNi0.33Co0.33Mn0.33O2 (NMC) electrodes made by a dry-powder-coating process. J. Electrochem. Soc. 2019, 166, A2151.
- Park, D.W.; Cañas, N.A.; Wagner, N.; Friedrich, K.A. Novel solvent-free direct coating process for battery electrodes and their electrochemical performance. J. Power Sources 2016, 306, 758–763.
- Helmers, L.; Froböse, L.; Friedrich, K.; Steffens, M.; Kern, D.; Michalowski, P.; Kwade, A. Sustainable Solvent-Free Production and Resulting Performance of Polymer Electrolyte-Based All-Solid-State Battery Electrodes. Energy Technol. 2021, 9, 2000923.
- Gyulai, A.; Bauer, W.; Ehrenberg, H. Dry Electrode Manufacturing in a Calender: The Role of Powder Premixing for Electrode Quality and Electrochemical Performance. ACS Appl. Energy Mater. 2023, 6, 5122–5134.
- Tao, R.; Steinhoff, B.; Sawicki, C.H.; Sharma, J.; Sardo, K.; Bishtawi, A.; Li, J. Unraveling the impact of the degree of dry mixing on dry-processed lithium-ion battery electrodes. J. Power Sources 2023, 580, 233379.
- Bauer, W.; Nötzel, D.; Wenzel, V.; Nirschl, H. Influence of dry mixing and distribution of conductive additives in cathodes for lithium ion batteries. J. Power Sources 2015, 288, 359–367.
- Bockholt, H.; Haselrieder, W.; Kwade, A. Intensive powder mixing for dry dispersing of carbon black and its relevance for lithium-ion battery cathodes. Powder Technol. 2016, 297, 266–274.
- Nam, Y.J.; Oh, D.Y.; Jung, S.H.; Jung, Y.S. Toward practical all-solid-state lithium-ion batteries with high energy density and safety: Comparative study for electrodes fabricated by dry-and slurry-mixing processes. J. Power Sources 2018, 375, 93–101.
- Wenzel, V.; Nirschl, H.; Nötzel, D. Challenges in lithium-ion-battery slurry preparation and potential of modifying electrode structures by different mixing processes. Energy Technol. 2015, 3, 692–698.
- Yang, J.; Sliva, A.; Banerjee, A.; Dave, R.N.; Pfeffer, R. Dry particle coating for improving the flowability of cohesive powders. Powder Technol. 2005, 158, 21–33.
- Wenzel, V.; Moeller, R.S.; Nirschl, H. Influence of Mixing Technology and the Potential to Modify the Morphological Properties of Materials used in the Manufacture of Lithium-Ion Batteries. Energy Technol. 2014, 2, 176–182.
- Li, Y.; Wu, Y.; Wang, Z.; Xu, J.; Ma, T.; Chen, L.; Wu, F. Progress in solvent-free dry-film technology for batteries and supercapacitors. Mater. Today 2022, 55, 92–109.
- Mitchell, P.; Zhong, L.; Xi, X.; Zou, B. Dry Particle Based Adhesive and Dry Film and Methods of Making Same. U.S. Patent US20200152987A1, 14 May 2020.
- Zou, B.; Zhong, L.; Mitchell, P.; Xi, X. Dry-Particle Packaging Systems and Methods of Making Same. U.S. Patent US20060137158A1, 29 June 2006.
- Mitchell, P.; Zhong, L.; Xi, X. Recyclable Dry Particle Based Adhesive Electrode and Methods of Making Same. U.S. Patent US7342770B2, 11 March 2008.
- Wu, Y.; Wang, S.; Li, H.; Chen, L.; Wu, F. Progress in thermal stability of all-solid-state-Li-ion-batteries. InfoMat 2021, 3, 827–853.
- Lu, P.; Liu, L.; Wang, S.; Xu, J.; Peng, J.; Yan, W.; Wang, Q.; Li, H.; Chen, L.; Wu, F. Superior all-solid-state batteries enabled by a gas-phase-synthesized sulfide electrolyte with ultrahigh moisture stability and ionic conductivity. Adv. Mater. 2021, 33, 2100921.
- Zhang, Z.; Wu, L.; Zhou, D.; Weng, W.; Yao, X. Flexible sulfide electrolyte thin membrane with ultrahigh ionic conductivity for all-solid-state lithium batteries. Nano Lett. 2021, 21, 5233–5239.
- Jiang, T.; He, P.; Wang, G.; Shen, Y.; Nan, C.W.; Fan, L.Z. Solvent-free synthesis of thin, flexible, nonflammable garnet-based composite solid electrolyte for all-solid-state lithium batteries. Adv. Energy Mater. 2020, 10, 1903376.
- Thieme, S.; Brückner, J.; Bauer, I.; Oschatz, M.; Borchardt, L.; Althues, H.; Kaskel, S. High capacity micro-mesoporous carbon–sulfur nanocomposite cathodes with enhanced cycling stability prepared by a solvent-free procedure. J. Mater. Chem. A 2013, 1, 9225–9234.
- Yao, W.; Chouchane, M.; Li, W.; Bai, S.; Liu, Z.; Li, L.; Sayahpour, B.; Shimizu, R.; Raghavendran, G.; Meng, Y.S. A 5 V-class cobalt-free battery cathode with high loading enabled by dry coating. Energy Environ. Sci. 2023, 16, 1620–1630.
- Zhang, Y.; Lu, S.; Lou, F.; Yu, Z. Solvent-free lithium iron phosphate cathode fabrication with fibrillation of polytetrafluoroethylene. Electrochim. Acta 2023, 456, 142469.
- Zhang, Y.; Huld, F.; Lu, S.; Jektvik, C.; Lou, F.; Yu, Z. Revisiting polytetrafluorethylene binder for solvent-free lithium-ion battery anode fabrication. Batteries 2022, 8, 57.
- Giles, H.F., Jr.; Mount, E.M., III; Wagner, J.R., Jr. Extrusion: The Definitive Processing Guide and Handbook; William Andrew: Norwich, UK, 2004.
- Crawford, D.E.; Casaban, J. Recent developments in mechanochemical materials synthesis by extrusion. Adv. Mater. 2016, 28, 5747–5754.
- Thiry, J.; Krier, F.; Evrard, B. A review of pharmaceutical extrusion: Critical process parameters and scaling-up. Int. J. Pharm. 2015, 479, 227–240.
- Dreger, H.; Haselrieder, W.; Kwade, A. Influence of dispersing by extrusion and calendering on the performance of lithium-ion battery electrodes. J. Energy Storage 2019, 21, 231–240.
- Gueguen, M.; Billion, M.; Majastre, H. Method of Manufacturing a Multilayer Electrochemical Assembly Comprising an Electrolyte between Two Electrodes, and an Assembly Made Thereby. U.S. Patent US5593462A, 10 September 1997.
- Jardiel, T.; Sotomayor, M.E.; Levenfeld, B.; Várez, A. Optimization of the Processing of 8-YSZ Powder by Powder Injection Molding for SOFC Electrolytes. Int. J. Appl. Ceram. Technol. 2008, 5, 574–581.
- Verdier, N.; Foran, G.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Dollé, M. Challenges in solvent-free methods for manufacturing electrodes and electrolytes for lithium-based batteries. Polymers 2021, 13, 323.
- Sotomayor, M.E.; de La Torre-Gamarra, C.; Bucheli, W.; Amarilla, J.M.; Varez, A.; Levenfeld, B.; Sanchez, J.Y. Additive-free Li4Ti5O12 thick electrodes for Li-ion batteries with high electrochemical performance. J. Mater. Chem. A 2018, 6, 5952–5961.
- Sotomayor, M.E.; de La Torre-Gamarra, C.; Levenfeld, B.; Sanchez, J.Y.; Varez, A.; Kim, G.T.; Passerini, S. Ultra-thick battery electrodes for high gravimetric and volumetric energy density Li-ion batteries. J. Power Sources 2019, 437, 226923.
- de La Torre-Gamarra, C.; Sotomayor, M.E.; Sanchez, J.Y.; Levenfeld, B.; Várez, A.; Laïk, B.; Pereira-Ramos, J.P. High mass loading additive-free LiFePO4 cathodes with 500 μm thickness for high areal capacity Li-ion batteries. J. Power Sources 2020, 458, 228033.
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Anode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating It. U.S. Patent 9,112,200, 18 August 2015.
- Voillequin, B.; Ayme-Perrot, D.; Dufour, B.; Sonntag, P. Cathode for a Cell of a Lithium-Ion Battery, Its Manufacturing Process and the Battery Incorporating it. U.S. Patent 9,484,571, 1 November 2016.
- El Khakani, S.; Verdier, N.; Lepage, D.; Prébé, A.; Aymé-Perrot, D.; Rochefort, D.; Dollé, M. Melt-processed electrode for lithium-ion battery. J. Power Sources 2020, 454, 227884.
- Grant, P.S.; Greenwood, D.; Pardikar, K.; Smith, R.; Entwistle, T.; Middlemiss, L.A.; Cumming, D.J. Roadmap on Li-ion battery manufacturing research. J. Phys. Energy 2022, 4, 042006.
- Zhang, Y.; Lu, S.; Wang, Z.; Volkov, V.; Lou, F.; Yu, Z. Recent technology development in solvent-free electrode fabrication for lithium-ion batteries. Renew. Sustain. Energy Rev. 2023, 183, 113515.
- Hyvärinen, M.; Jabeen, R.; Kärki, T. The modelling of extrusion processes for polymers—A review. Polymers 2020, 12, 1306.
- Zhou, H.; Liu, M.; Gao, H.; Hou, D.; Yu, C.; Liu, C.; Zhang, D.; Wu, J.C.; Yang, J.; Chen, D. Dense integration of solvent-free electrodes for Li-ion supercabattery with boosted low temperature performance. J. Power Sources 2020, 473, 228553.
- Astafyeva, K.; Dousset, C.; Bureau, Y.; Stalmach, S.L.; Dufour, B. High Energy Li-Ion Electrodes Prepared via a Solventless Melt Process. Batter. Supercaps 2020, 3, 341–343.




