
| Version | Summary | Created by | Modification | Content Size | Created at | Operation |
|---|---|---|---|---|---|---|
| 1 | Pranjal K. Baruah | -- | 3019 | 2024-02-07 07:39:51 | | | |
| 2 | Rita Xu | -108 word(s) | 2911 | 2024-02-07 07:56:28 | | |
Video Upload Options
Antimicrobial resistance (AMR) has become an alarming threat to the successful treatment of rapidly growing bacterial infections because of the abuse and misuse of antibiotics. Traditional antibiotics bear many limitations including restricted bioavailability, inadequate penetration and the emergence of antimicrobial-resistant microorganisms. Recent advances in nanotechnology for the introduction of nanoparticles with fascinating physicochemical characteristics have been predicted as an innovative means of defence against antimicrobial-resistant diseases. The use of nanoparticles renders several benefits including improved tissue targeting, better solubility, improved stability, enhanced epithelial permeability and minimal side effects.
1. Introduction
2. AMR—A Global Threat
3. Bacterial Resistance Mechanisms
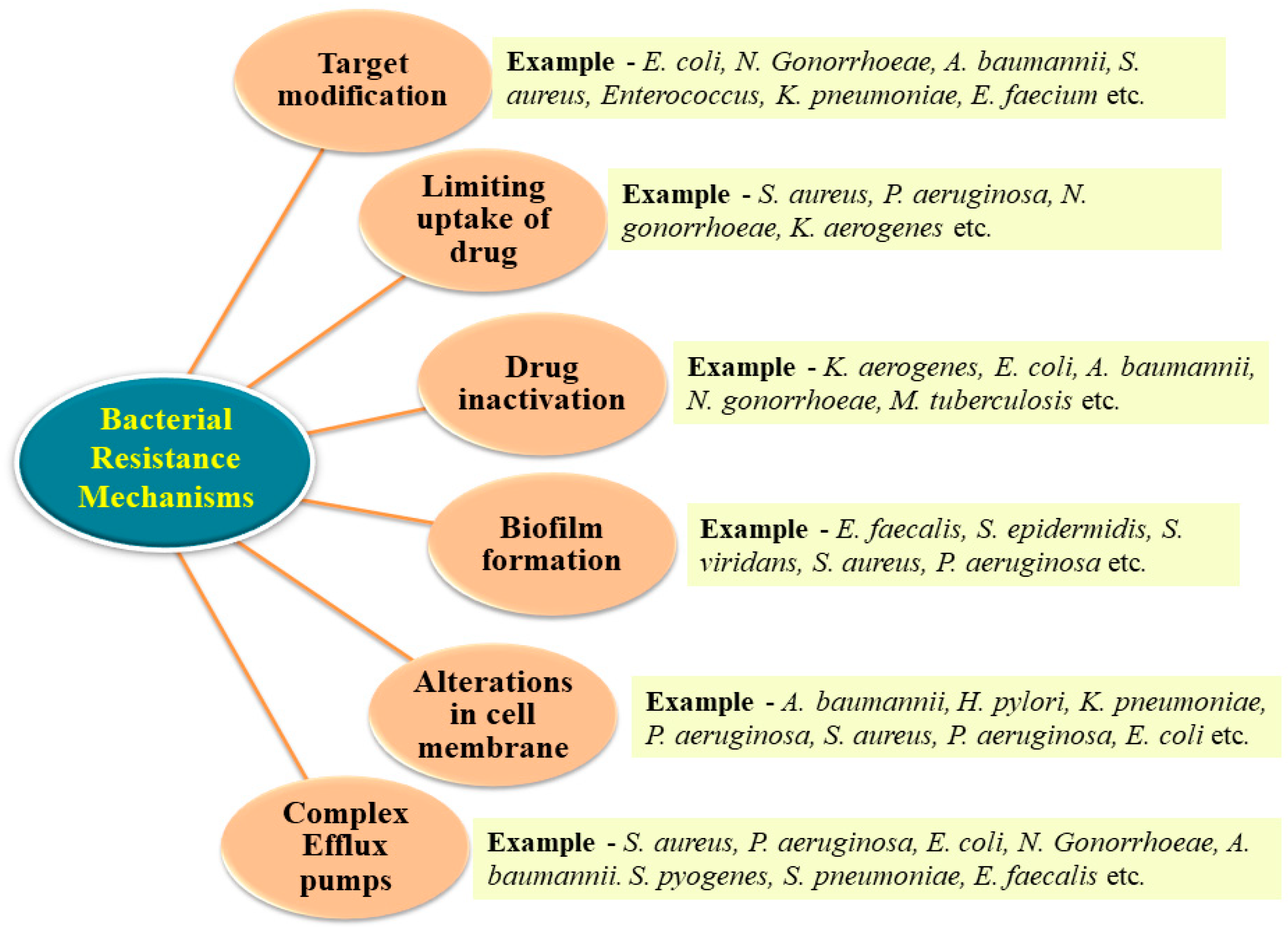
3.1. Inactivation of the Drug
3.2. Target Modification
3.3. Limiting Drug Uptake
3.4. Development of Efflux Pump
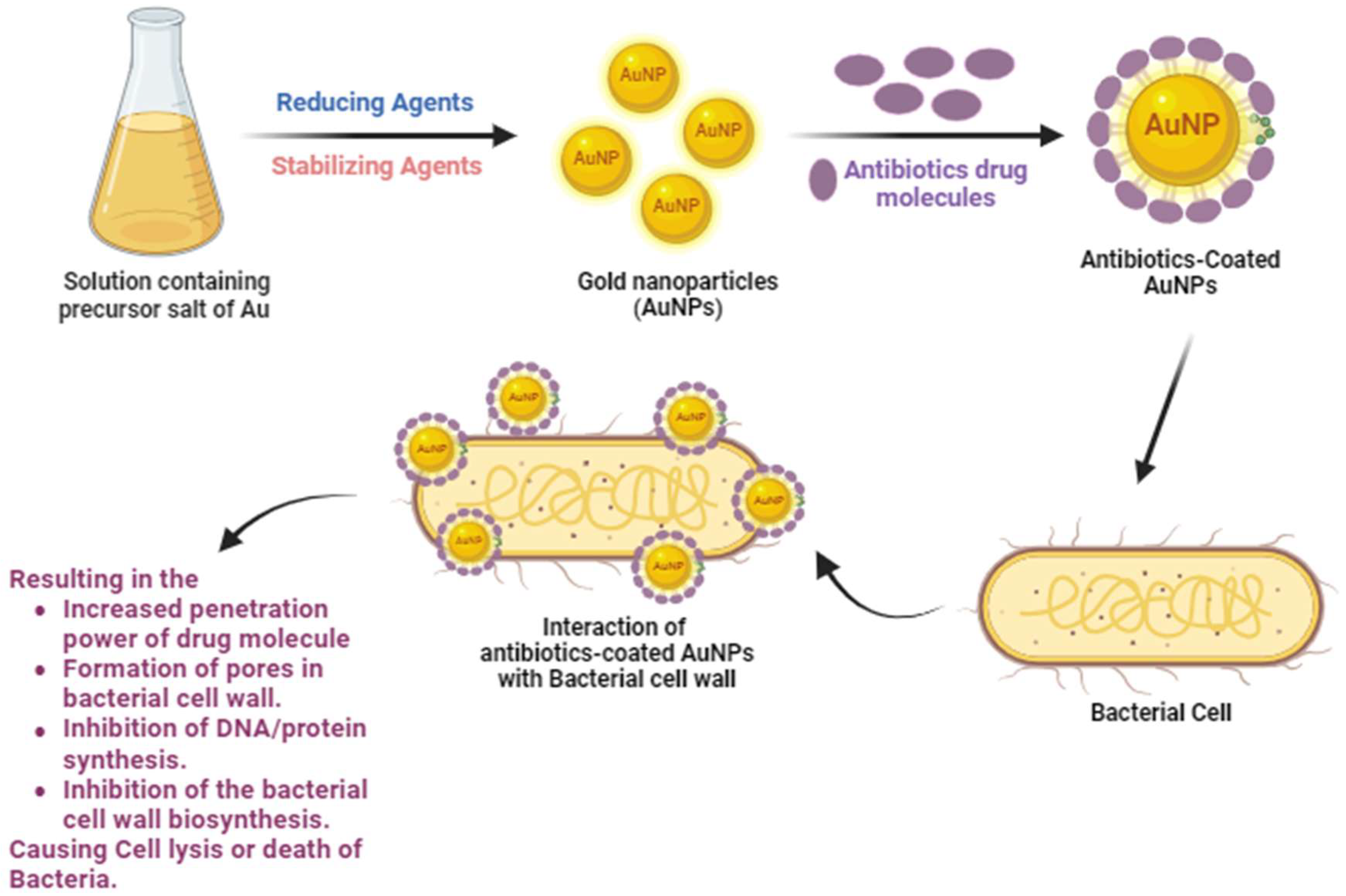
4. Synthesis of Antibiotic-Coated AuNPs
References
- Khandelwal, P.; Singh, D.K.; Poddar, P. Advances in the Experimental and Theoretical Understandings of Antibiotic Conjugated Gold Nanoparticles for Antibacterial Applications. ChemistrySelect 2019, 4, 6719–6738.
- Davies, J.; Davies, D. Origins and Evolution of Antibiotic Resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433.
- Kalhapure, R.S.; Suleman, N.; Mocktar, C.; Seedat, N.; Govender, T. Nanoengineered Drug Delivery Systems for Enhancing Antibiotic Therapy. J. Pharm. Sci. 2015, 104, 872–905.
- Cars, O.; Hedin, A.; Heddini, A. The global need for effective antibiotics—Moving towards concerted action. Drug Resist. Updates 2011, 14, 68–69.
- Ali, S.; Perveen, S.; Shah, M.R.; Zareef, M.; Arslan, M.; Basheer, S.; Ullah, S.; Ali, M. Bactericidal potentials of silver and gold nanoparticles stabilized with cefixime: A strategy against antibiotic-resistant bacteria. J. Nanoparticle Res. 2020, 22, 201.
- Rocca, D.M.; Silvero, C.M.J.; Aiassa, V.; Cecilia Becerra, M. Rapid and effective photodynamic treatment of biofilm infections using low doses of amoxicillin-coated gold nanoparticles. Photodiagn. Photodyn. Ther. 2020, 31, 101811.
- Rizvi, S.M.; Lila, A.S.; Moin, A.; Hussain, T.; Kamal, M.A.; Sonbol, H.; Khafagy, E.-S. Antibiotic-Loaded Gold Nanoparticles: A Nano-Arsenal against ESBL Producer-Resistant Pathogens. Pharmaceutics 2023, 15, 430.
- Teklu, D.S.; Negeri, A.A.; Legese, M.H.; Bedada, T.L.; Woldemariam, H.K.; Tullu, K.D. Extended-spectrum beta-lactamase production and multi-drug resistance among Enterobacteriaceae isolated in Addis Ababa, Ethiopia. Antimicrob. Resist. Infect. Control 2019, 8, 39.
- Saha, M.; Sarkar, A. Review on Multiple Facets of Drug Resistance: A Rising Challenge in the 21st Century. J. Xenobiotics 2021, 11, 197–214.
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318.
- Haddada, M.B.; Jeannot, K.; Spadavecchia, J. Novel Synthesis and Characterization of Doxycycline-loaded Gold Nanoparticles: The Golden Doxycycline for Antibacterial Applications. Part. Part. Syst. Charact. 2018, 36, 1800395.
- Hakemi-Vala, M.; Rafati, H.; Aliahmadi, A.; Ardalan, A. Nanoemulsions: A Novel Antimicrobial Delivery System. In Nano-and Microscale Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2017; pp. 245–266.
- Yeh, Y.C.; Huang, T.H.; Yang, S.C.; Chen, C.C.; Fang, J.Y. Nano-Based Drug Delivery or Targeting to Eradicate Bacteria for Infection Mitigation: A Review of Recent Advances. Front. Chem. 2020, 8, 286.
- Pizzolato-Cezar, L.R.; Okuda-Shinagawa, N.M.; Machini, M.T. Combinatory Therapy Antimicrobial Peptide-Antibiotic to Minimize the Ongoing Rise of Resistance. Front. Microbiol. 2019, 10, 1703.
- Khandelwal, P.; Poddar, P. Fluorescent metal quantum clusters: An updated overview of the synthesis, properties, and biological applications. J. Mater. Chem. B 2017, 5, 9055–9084.
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria “a Battle of the Titans”. Front. Microbiol. 2018, 9, 1441.
- Gholipourmalekabadi, M.; Mobaraki, M.; Ghaffari, M.; Zarebkohan, A.; Omrani, V.; Urbanska, A.; Seifalian, A. Targeted Drug Delivery Based on Gold Nanoparticle Derivatives. Curr. Pharm. Des. 2017, 23, 20.
- Wang, Z.; Dong, K.; Liu, Z.; Zhang, Y.; Chen, Z.; Sun, H.; Ren, J.; Qu, X. Activation of biologically relevant levels of reactive oxygen species by au/g-c3n4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials 2017, 113, 145–157.
- Zhao, Y.; Jiang, X. Multiple strategies to activate gold nanoparticles as antibiotics. Nanoscale 2013, 5, 8340.
- Wadhwani, P.; Heidenreich, N.; Podeyn, B.; Bürck, J.; Ulrich, A.S. Antibiotic gold: Tethering of antimicrobial peptides to gold nanoparticles maintains conformational flexibility of peptides and improves trypsin susceptibility. Biomater. Sci. 2017, 5, 817–827.
- Hussein, M.A.; Grinholc, M.; Dena, A.S.; El-Sherbiny, I.M.; Megahed, M. Boosting the antibacterial activity of chitosan–gold nanoparticles against antibiotic–resistant bacteria by Punicagranatum L. Extract. Carbohydr. Polym. 2021, 256, 117498.
- Sinha, R.; Karan, R.; Sinha, A.; Khare, S.K. interaction and nanotoxic effect of zno and ag nanoparticles on mesophilic and halophilic bacterial cells. Bioresour. Technol. 2011, 102, 1516–1520.
- Hemeg, H. Nanomaterials for alternative antibacterial therapy. Int. J. Nanomed. 2017, 12, 8211–8225.
- Dakal, T.C.; Kumar, A.; Majumdar, R.S.; Yadav, V. Mechanistic Basis of Antimicrobial Actions of Silver Nanoparticles. Front. Microbiol. 2016, 7, 1831.
- Sarma, P.P.; Barman, K.; Baruah, P.K. Green synthesis of silver nanoparticles using Murraya koenigii leaf extract with efficient catalytic, antimicrobial, and sensing properties towards heavy metal ions. Inorg. Chem. Commun. 2023, 152, 110676.
- Manju, S.; Malaikozhundan, B.; Vijayakumar, S.; Shanthi, S.; Jaishabanu, A.; Ekambaram, P.; Vaseeharan, B. Antibacterial, antibiofilm and cytotoxic effects of Nigella sativa essential oil coated gold nanoparticles. Microb. Pathog. 2016, 91, 129–135.
- Okkeh, M.; Bloise, N.; Restivo, E.; De Vita, L.; Pallavicini, P.; Visai, L. Gold Nanoparticles: Can They Be the next Magic Bullet for Multidrug-Resistant Bacteria? Nanomaterials 2021, 11, 312.
- Khanna, S.; Padhan, P.; Das, S.; Jaiswal, K.S.; Tripathy, A.; Smita, S.; Tripathy, S.K.; Raghav, S.K.; Gupta, B. A Simple Colorimetric Method for Naked-eye Detection of Circulating Cell-free DNA Using Unlabelled Gold Nanoparticles. ChemistrySelect 2018, 3, 11541–11551.
- Kukreja, A.; Kang, B.; Kim, H.O.; Jang, E.; Son, H.Y.; Huh, Y.M.; Haam, S. Preparation of gold core-mesoporous iron-oxide shell nanoparticles and their application as dual MR/CT contrast agent in human gastric cancer cells. J. Ind. Eng. Chem. 2017, 48, 56–65.
- Ahangari, A.; Salouti, M.; Saghatchi, F. Gentamicin-gold nanoparticles conjugate: A contrast agent for x-ray imaging of infectious foci due to Staphylococcus aureus. IET Nanobiotechnol. 2016, 10, 190–194.
- Pourali, P.; Yahyaei, B.; Afsharnezhad, S. Bio-Synthesis of Gold Nanoparticles by Fusarium oxysporum and Assessment of Their Conjugation Possibility with Two Types of β-Lactam Antibiotics without Any Additional Linkers. Microbiology 2018, 87, 229–237.
- Alafnan, A.; Rizvi, S.M.; Alshammari, A.S.; Faiyaz, S.S.; Lila, A.S.; Katamesh, A.A.; Khafagy, E.S.; Alotaibi, H.F.; Ahmed, A.B. Gold Nanoparticle-Based Resuscitation of Cefoxitin against Clinical Pathogens: A Nano-Antibiotic Strategy to Overcome Resistance. Nanomaterials 2022, 12, 3643.
- Zong, T.-X.; Silveira, A.P.; Morais, J.A.; Sampaio, M.C.; Muehlmann, L.A.; Zhang, J.; Jiang, C.-S.; Liu, S.-K. Recent Advances in Antimicrobial Nano-Drug Delivery Systems. Nanomaterials 2022, 12, 1855.
- Tayeb, H.H.; Moqaddam, S.A.; Hasaballah, N.H.; Felimban, R.I. Development of nanoemulsions for the delivery of hydrophobic and hydrophilic compounds against carbapenem-resistant Klebsiella pneumoniae. RSC Adv. 2022, 12, 26455–26462.
- Larsson, D.G.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2021, 20, 257–269.
- Sengupta, S.; Chattopadhyay, M.K.; Grossart, H. The multifaceted roles of antibiotics and antibiotic resistance in nature. Front. Microbiol. 2013, 4, 47.
- Alba, C.; Blanco, A.; Alarcon, T. Antibiotic resistance in Helicobacter pylori. Curr. Opin. Infect. Dis. 2017, 30, 489–497.
- Kohler, V.; Vaishampayan, A.; Grohmann, E. Problematic Groups of Multidrug-Resistant Bacteria and Their Resistance Mechanisms. In Antibacterial Drug Discovery to Combat MDR; Springer: Berlin/Heidelberg, Germany, 2019; pp. 25–69.
- Chaudhuri, A.; Martin, P.M.; Kennedy, P.G.; Andrew Seaton, R.; Portegies, P.; Bojar, M.; Steiner, I. EFNS guideline on the management of community-acquired bacterial meningitis: Report of an EFNS Task Force on acute bacterial meningitis in older children and adults. Eur. J. Neurol. 2008, 15, 649–659.
- Poulin-Laprade, D.; Matteau, D.; Jacques, P.E.; Rodrigue, S.; Burrus, V. Transfer Activation of SXT/R391 integrative and conjugative elements: Unraveling the SetCD Regulon. Nucleic Acids Res. 2015, 43, 2045–2056.
- Afshinnekoo, E.; Bhattacharya, C.; Burguete-García, A.; Castro-Nallar, E.; Deng, Y.; Desnues, C.; Dias-Neto, E.; Elhaik, E.; Iraola, G.; Jang, S.; et al. COVID-19 drug practices risk antimicrobial resistance evolution. Lancet Microbe 2021, 2, E135–E136.
- Blair, J.M.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2014, 13, 42–51.
- Chawla, M.; Verma, J.; Gupta, R.; Das, B. Antibiotic Potentiators against Multidrug-Resistant Bacteria: Discovery, Development, and Clinical Relevance. Front. Microbiol. 2022, 13, 887251.
- Blair, J.M.; Richmond, G.E.; Piddock, L.J. Multidrug Efflux Pumps in Gram-Negative Bacteria and Their Role in Antibiotic Resistance. Future Microbiol. 2014, 9, 1165–1177.
- Villagra, N.A.; Fuentes, J.A.; Jofre, M.R.; Hidalgo, A.A.; Garcia, P.; Mora, G.C. The carbon source influences the efflux pump-mediated antimicrobial resistance in clinically important Gram-negative bacteria. J. Antimicrob. Chemother. 2012, 67, 921–927.
- Samanta, S.; Agarwal, S.; Nair, K.K.; Harris, R.A.; Swart, H. Biomolecular assisted synthesis and mechanism of silver and gold nanoparticles. Mater. Res. Express 2019, 6, 82009.
- Barman, K.; Chowdhury, D.; Baruah, P.K. Bio-synthesized silver nanoparticles using Zingiber officinale rhizome extract as efficient catalyst for the degradation of environmental pollutants. Inorg. Nano-Met. Chem. 2019, 50, 57–65.
- Li, N.; Zhao, P.; Astruc, D. Anisotropic Gold Nanoparticles: Synthesis, Properties, Applications, and Toxicity. Angew. Chem. 2014, 53, 1756–1789.
- Fan, Y.; Pauer, A.C.; Gonzales, A.A.; Fenniri, H. Enhanced antibiotic activity of ampicillin conjugated to gold nanoparticles on PEGylated rosette nanotubes. Int. J. Nanomed. 2019, 14, 7281–7289.
- Halawani, E.M.; Alzahrani, S.S.; Gad El-Rab, S.M. Biosynthesis Strategy of Gold Nanoparticles and Biofabrication of a Novel Amoxicillin Gold Nanodrug to Overcome the Resistance of Multidrug-Resistant Bacterial Pathogens MRSA and E. Coli. Biomimetics 2023, 8, 452.





