1. Introduction
The tremendous increase in antibiotic resistance among pathogenic microorganisms is a major hazard to human wellness. Antibiotics can be regarded as the organic compounds that suppress the growth of or kill bacterial cells, thereby preventing infections caused by pathogenic bacteria
[1][2][1,2]. Antibiotics are considered as vital in practically every major therapeutic field, including serious surgeries, notably the transplantation of organs, premature newborn treatment, and chemotherapy medication in cancer patients, which could not be achieved without adequately avoiding and treating bacterial infections
[3][4][3,4]. The most frequently used antibacterial medications are β-lactam antibiotics; yet, because of growing bacterial resistance, several drugs of this class have loosened their clinical effectiveness
[5]. Furthermore, using antibiotics at higher doses during treatment promotes the emergence of multidrug resistance (MDR), rendering antibiotic therapy ineffective against pathogenic bacteria
[6]. However, another important factor that contributes to the development of MDR in bacteria is the uses of sub-lethal doses of antibiotics, wherein bacteria gain resistance to drugs without being killed. Incorrect and excessive antibiotic administration, insufficient diagnosis, and advanced resistance mechanisms accrued by microorganisms can all lead to collapses of therapeutic efficiency
[7][8][7,8]. Biofilm-associated illnesses which have become tolerant to current antibiotic treatments leading to scarcity of efficient therapeutic alternatives have become a serious matter of concern nowadays. Besides these, the other contributing factors for increased AMR include the widespread use of antibiotics in the field of agriculture as well as in dairy and poultry industries, a lack of novel medications, massive regulatory hurdles, incorrect prescriptions, etc.
[9][10][9,10]. The inadequate effectiveness of traditional antibiotics, whether due to extended-spectrum β-lactamases (ESBLs) or through other resistance pathways, necessitates the innovation of novel therapeutic remedies with enhanced activities. Furthermore, combating infections using as low a dose as possible might serve as the most efficient approach to fight against diseases caused by MDR bacteria
[7]. While developing new antimicrobial compounds to fight drug-resistant bacteria is extremely difficult, it is also extremely crucial and necessary in the interim. Chemical alteration in antibiotic structures to combat antimicrobial resistance as well as the synthesis of novel antibiotics with high efficiency is arduous and is typically not economically viable. To render antibiotic therapy more efficient and cost-effective, the antibiotic dose must be reduced while the stability must be enhanced
[11].
Recent advances in medical research introduced novel antibiotics and advanced clinical therapies that can effectively tackle pathogenic microorganisms
[12]. The application of nanomaterials in the domain of antibiotic drug delivery offers the potential for significant improvements in the clinical effectiveness of antibacterial treatment. In comparison to traditional medications, nanoformulations for the administration of antibiotics along with infectious site targeting provide several advantages, which comprise enhanced tissue targeting, prolonged antibiotic half-life, raised solubility, gained greater stability, increased permeability to epithelial cells, and limited side effects
[13][14][13,14]. Recently, nanoparticles (NPs) (having a particle size between 1 and 100 nm) have come into the limelight as a revolutionary approach for treating deadly diseases caused by bacteria. Moreover, metallic NPs functionalised with antibiotics offer promising nanoplatforms for fighting against bacterial resistance.
The use of nanomaterials in medicine has also historical importance in India. Bhasmas, which have been utilised as Ayurvedic medicines in India to cure a variety of diseases including cancer, were discovered to contain NPs of alloys, sulphides, metals and metal oxides
[1][15][1,15]. Nanomaterials possess promising potential to be used in the field of drug delivery, medical imaging and disease diagnosis, considering their high surface area as well as their small size effect. Among the diverse range of nanomaterials, NPs like AgNPs, AuNPs, ZnONPs and CuONPs have been receiving specific attention because of their easy and simple synthesis procedure, higher biocompatibility and versatile physicochemical properties that can be easily altered by stimulating parameters like temperature, pH, light and reaction time
[16][17][18][16,17,18]. NPs exhibit the tremendous potential to serve as drug carriers and can be easily functionalised by incorporating bioactive compounds, drug molecules, antibiotics, polymers, antibacterial peptides, etc. for the enhancement of antimicrobial activity to combat AMR
[19][20][21][19,20,21]. Polymer-functionalised NPs have offered immense promises in the field of novel drug carrier and delivery systems owing to their great potential for protecting as well as improving the bioavailability and release rate of encapsulated drug molecules thereby contributing to the reduction in toxic impacts
[21]. Because of their flexible physical characteristics, NPs serve as a versatile system for medicinal applications. The small size of the nanomaterials imparts a high surface-to-volume ratio, which promotes the binding of several antibacterial agents in order to produce multivalent nanomaterials against pathogenic bacteria
[7]. More importantly, many NPs demonstrate inherent antimicrobial characteristics by inhibiting biofilm formation, activating reactive oxygen production, and interfering with bacterial cell membranes as well as with proteins and DNA within the bacterial cell
[16]. Several metal NPs, such as Ag, Au, Cu, Zn, Ce, Mg, Pd, Ti, etc., and metal oxide NPs, such as ZnO, CuO, NiO, Al
2O
3 TiO and Fe
3O
2, as well as their functionalisation through conjugation with other compounds, have been reported to demonstrate antibacterial properties against various pathogenic bacteria
[16][22][23][24][25][16,22,23,24,25]. Compared to the pure AuNPs, the conjugated or functionalised AuNPs demonstrate better antimicrobial properties
[19][20][21][19,20,21]. Since NPs do not exhibit a specific mechanism of action unlike antibiotics, these are especially advantageous in combating bacterial resistance
[1]. Recent studies revealed that AuNPs do not impart any toxic effects in human cells; hence, AuNPs have captured the interest of modern biomedical researchers
[26]. AgNPs are also used in medicinal therapies. Although AgNPs are easy to synthesise, cost effective and possess inherent antimicrobial property, their cytotoxicity due to the release as well as aggregation of Ag ions in the body from AgNPs has restricted their utilisation in nanomedicine. However, the higher biocompatibility ease of synthesis and inertness of the AuNPs make them suitable for use in medicinal therapies. High cost is the major disadvantage associated with AuNPs
[27][28][27,28]. The higher biocompatibility, nontoxicity, high rates of absorption and powerful light scattering allow them to be used in versatile fields, such as electronics, sensors, catalysis, drug delivery, drug carriers and other biomedical applications, including delivery of genes, molecular imaging, targeted drug administration, plasmonic bio-sensing, tissue engineering, colorimetric sensing, cancer treatment, diagnostics and photo-induced therapy
[27][28][29][27,28,29]. Recently, AuNPs were employed to administer numerous antibiotics belonging to different classes that included carbapenems, polymyxins, tetracycline, cephalosporin, glycopeptide, cephalosporin, aminoglycoside, penicillin and cephalosporin
[1][30][31][1,30,31]. However, numerous limitations are associated with modern as well as conventional antibiotic medication therapy, including limited bioavailability, the emergence of antibiotic-resistant bacteria, poor penetration power, insufficient drug concentrations at specific infection sites, adverse side effects, a higher frequency of administration, and poor patient compliance. Therefore, AuNP-conjugated drug delivery systems provide a superior and revolutionary approach to eradicating the disadvantages associated with the use of antimicrobial drug therapy, because of their large surface-to-volume ratio, photostability, ability to target biofilms, potential for organ as well as cellular targeting, strong interaction and their penetration through the bacterial cell wall
[3][13][3,13]. The conjugation of antibiotic drug molecules with AuNPs significantly enhances their antimicrobial properties. The conjugation of antibiotic drugs with AuNPs leads to the enhanced bioavailability and biocompatibility of the drugs. This can also enhance the interaction as well as penetration power of the drug molecules though the bacterial cell wall. The electrostatic attraction between the negatively charged bacterial cell wall and the cationic behaviour of the antibiotic-loaded AuNPs enhances the interaction as well as penetrating power of the drug molecules through the bacterial cell wall. This facilitates the release of the antibiotic drug molecules within the bacterial cell and interacts with the bacterial cellular matrix causing the death of bacteria
[5][6][5,6]. Indeed, AuNPs are effective for the transformation of various ineffective antibiotics into Au nanoformulations with strong antimicrobial properties
[32]. However, the efficient incorporation of a specific antibiotic drug molecule on the surface of the AuNPs requires a variety of strategies, including physical absorption, electrostatic relations, coupling processes and Au-S and Au-N linkages
[1]. Different physical, chemical and biological methods, including green synthesis, for the fabrication of AuNPs have been reported. Among these, chemical colloidal synthesis, which involves the use of a metal precursor as well as a reducing and a stabilising agent, is one of the most frequently employed methods for the synthesis of AuNPs
[1][7][1,7].
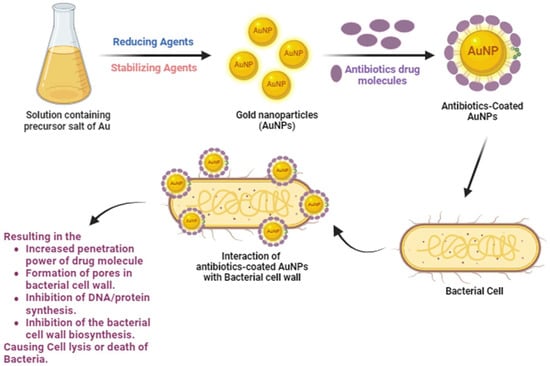
Special emphasis has been put on the development of bioactive and antibiotic-coated AuNPs exhibiting enhanced antimicrobial attributes to combat pathogenic bacteria and biofilms, as well as their activities and physicochemical features. A schematic representation of the synthesis of antibiotic-coated AuNPs and their interaction with bacterial cells to combat AMR is demonstrated in
Figure 1.
Figure 1. Synthesis of antibiotic-coated AuNPs and their interaction with the bacterial cell to combat AMR.
2. AMR—A Global Threat
The World Health Organization (WHO) has recognised AMR as one of the leading global hazards to human health and development. It falls among the top ten public health threats worldwide
[33]. The escalating growth and dissemination of antibiotic-resistant organisms and the inadequate efficacy of antimicrobial drugs make the treatment of antimicrobial infection challenging, leading to elevated fatality rates and huge monetary expenses
[34][35][34,35]. With the current scientific revolutions, antibiotics have become a wonderful gift to the livestock and human healthcare professions to aid in the cure of bacterial infections as well as other disorders. Before the 1950s, antibiotics were frequently employed in both human health and animal husbandry due to their low cost and few side effects
[13]. However, the serious problem of drug resistance has arisen as a result of the decades-long widespread use of antibiotics. The discovery of β-lactam antibiotics provided a temporary solution to the problem, but disappointingly, this did not endure for long with the first instance of methicillin-resistant
Staphylococcus aureus (MRSA) identified in the UK in 1961
[27][36][27,36]. The ability of a specific microbe to avoid the pharmacological mechanism of action linked to antibacterial medications and then go on living is known as antibacterial resistance. The growing number of drug-resistant microorganisms has been strongly attributed to the inappropriate application of broad-spectrum antibiotics.
Klebsiella pneumoniae is a multi-drug resistant (MDR) bacteria that causes several types of hospital-acquired illnesses and epidemics, including septicaemia, urinary tract infections and pneumonia
[14]. It has developed resistance against carbapenem and tigecycline, which is the most effective as well as a last-resort antibiotic for treating
K. pneumoniae. Due to the secretion of the β lactam enzymes like carbapenemases and extended-spectrum β-lactamases,
K. pneumoniae is capable of hydrolysing a wide range of broad-spectrum β-lactam antibiotics
[17][37][17,37].
Salmonella spp. responsible for diseases like bacteremia, gastroenteritis, paratyphoid and typhoid have become fluoroquinolone-resistant
[38]. Infections like pneumonia, tuberculosis and gonorrhoea become challenging to cure since medications are becoming less effective towards these diseases because of their increased resistance of bacteria. Moreover, ESBLs have now developed resistance to several antibiotic classes, including aminoglycosides, tetracyclines, cotrimoxazole, quinolones and trimethoprim, further limiting therapeutic choices for clinicians
[7][39][7,39]. Multidrug resistance has emerged as a global threat and is accelerating rapidly. Nowadays, MDR has become more prevalent in both pathogenic as well as non-pathogenic bacterial strains and is primarily attributed to gene acquisition and/or alteration in the target genes of antibiotics
[9]. Besides these, the antibiotic resistance of bacteria was regarded as one of the major economic burdens for the world
[9]. Lack of access to conventional antibiotics, however, will result in costlier medication, extended hospital exposure, prolonged treatment period, higher treatment expenses due to the need for intensive care and, ultimately, life-threatening harm
[40]. The widespread usage of antibiotics during the COVID-19 pandemic has fuelled the issue of antimicrobial resistance. Antibiotics, antiviral and antipyretic medications that have been used erratically to treat COVID-19 have led to the emergence of AMR
[41].
3. Bacterial Resistance Mechanisms
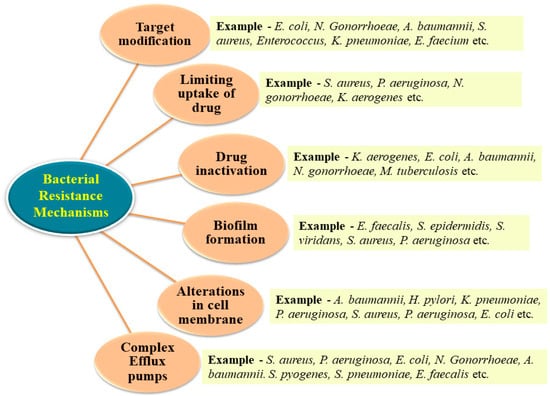
There are four primary mechanisms including the inactivation of drug molecules, target modification, limiting drug uptake and the development of efflux pumps through which bacteria can develop resistance towards antibiotics
[9][42][9,51]. Some strategies adopted by bacteria to develop resistance against antibiotics are represented in
Figure 2.
Figure 2. Bacterial approaches towards antibacterial resistance.
3.1. Inactivation of the Drug
In severe cases, bacteria acquire resistance through their direct interaction with the antibiotics via hydrolysis or the transfer of a chemical moiety including adenyl, acetyl and phosphoryl groups
[42][51]. A wide range of Gram-positive and Gram-negative bacteria develop resistance to certain antibiotics through the secretion of enzymes that have the ability to bring out chemical modifications to the antibacterial compound. Among these, β-lactamases are the most prominent, which can demolish the drug molecule by hydrolysing the amide linkage of the β-lactam ring, rendering it ineffective. The most common biochemical reactions catalysed by modifying enzymes include acetylation, phosphorylation and adenylation
[42][43][51,52].
3.2. Target Modification
Bacteria can modify the target of the antibiotic through protection, mutation and posttranslational modification
[27]. Point mutations within the gene generating the target site, enzymatic transformations of the target and evading the original site are the primary components of target site alteration. Evading the original site, bacteria create new targets that resemble the original but are not exact replicas of it. As a result, antibiotics will be unable to inhibit the metabolic processes accomplished by newly developed equivalent targets
[42][43][51,52].
3.3. Limiting Drug Uptake
The primary surface membrane component found in practically all Gram-negative bacteria is lipopolysaccharide (LPS), which is crucial to the outer membrane’s structure as well as its functionality
[42][44][51,53]. The presence of this LPS layer provides a barrier to a large number of antibacterial agents. However, for Gram-positive bacteria, the outer membrane is absent. The presence of the outer membrane in the Gram-negative bacteria is the primary reason for the development of resistance towards a wide range of antibiotics. Modifications in this outer membrane, such as the mutation of porins or the alteration of hydrophobic properties, can impart resistance
[42][44][51,53]. Hence, bacterial infections caused by Gram-negative bacteria are difficult to cure compared to Gram-positive bacterial infections. In Gram-negative bacteria, drug molecules penetrate the cell through porin channels. Bacteria can impede drug uptake by reducing the porin channels or by mutations that lead to alterations in the selectivity of the porin.
3.4. Development of Efflux Pump
Genes producing efflux pumps are found chromosomally in bacteria. Transmembrane proteins called efflux pumps enable bacteria to exchange or evacuate antibiotic compounds from their cells, which helps the bacteria to survive
[42][45][51,54]. These are the bacteria’s self-defence mechanisms. Based on structure as well as energy sources, there are five major families of efflux pumps, which include the ATP binding cassette family, small multidrug resistance family, resistance–nodulation–cell division family, multidrug and toxic compound extrusion family and major facilitator superfamily
[42][51].
4. Synthesis of Antibiotic-Coated AuNPs
A variety of physical, chemical or green synthesis techniques could be employed for the synthesis of AuNPs. The most frequently employed physical methods for the fabrication of AuNPs are laser ablation and evaporation–condensation
[46][55]. The fundamental steps in the chemical synthesis method of AuNPs involve the formation of Au
0 through the reduction of Au
+ ions by the action of a reducing agent followed by stabilisation via capping agents
[7]. During the chemical synthesis procedures, different chemicals were used as reducing and capping agents. The synthesis of AuNPs from vitamins, enzymes, microbes and plant extracts using green solvents is the prime focus of green synthesis methods. The green synthesized, antibiotic-loaded nanomaterials serve as a fascinating and significantly effective alternative for the treatment of disease caused by the microbes as they reduce or avoid the use of any hazardous compounds, thereby reducing side effects
[25][47][25,56]. AuNPs may exhibit various colours depending on their shape, size, aggregation level and surrounding environment because of the surface plasmon resonance. A variation of colour from light pink to dark purple can appear owing to the localised surface plasmon resonance (SPR), which is further associated with the size and shape of the NPs
[7][48][7,57]. AuNPs exhibit a SPR band between 500 and 550 nm
[48][57]. However, the facile surface functionalisation of AuNPs makes it easier to conjugate versatile bioactive compounds or ligands with AuNPs which significantly enhances the functionality of the NPs. The incorporation of antibiotic drug molecules on the surface of AuNPs is a recent innovation and is reported to be highly efficient in combating bacterial resistance toward traditional antibiotics. In most of the studies, the antibiotic-coated AuNPs were fabricated using chemical methods. Antibiotics are able to adhere to the surface of AuNPs by means of their different functional groups, including amino, carboxylic, hydroxyl and thiols, resulting in the surface modification of AuNPs leading to the enhancement in the antibacterial activity of the system
[49][58]. Temperature, reaction time and pH are the imminent factors that significantly affect the properties of the AuNPs. Recently, in 2023, Halawani et al. evaluated the effect of the concentration of precursor salt, temperature, reaction time and pH on the synthesis of amoxicillin-conjugated AuNPs
[50][59]. They have reported that with an increase in the concentration of the precursor salt from 1 mM to 5 mM, the absorption spectra become more intense, showing a blue shift and revealing the decreased size of the synthesized AuNPs. Further increasing the concentration beyond 5 mM leads to aggregation and precipitation of the NPs indicating a decrease in stability. Time also possesses a crucial role in the synthesis of NPs. An increase in the absorption peak was reported up to 1 h; however, further increasing the reaction time showed a decrease in the intensity of the absorption spectra. They have reported that the ideal pH for the synthesis of AuNPs is 6.